An In-depth Analysis of urapidil's R&D Progress and Mechanism of Action on Drug Target
Urapidil's R&D Progress
Urapidil is a small molecule drug that primarily targets the ADRA1 receptor. It has been approved for use in the treatment of various cardiovascular diseases, urogenital diseases, and other diseases. The drug is specifically indicated for the management of dysuria, urination disorders, and hypertension.
Urapidil has reached the highest phase of development which is approved globally. The drug was first approved in Germany in January 1980, making it a well-established medication in the pharmaceutical market.
As a small molecule drug, Urapidil is designed to interact with the ADRA1 receptor, which plays a crucial role in regulating blood pressure and urinary function. By targeting this receptor, Urapidil can effectively manage hypertension and related symptoms such as dysuria and urination disorders.
The approval of Urapidil in multiple therapeutic areas highlights its versatility and potential to address various medical conditions. Cardiovascular diseases, including hypertension, are a significant global health concern, affecting millions of individuals worldwide. Urogenital diseases, such as dysuria and urination disorders, can also significantly impact a person's quality of life. By targeting these conditions, Urapidil offers a promising treatment option for patients suffering from these ailments.
Since its initial approval in Germany in 1980, Urapidil has gained recognition and approval in other countries, including China. This indicates its efficacy and safety profile, as well as its potential to address the unmet medical needs of patients in different regions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for urapidil: ADRA1 antagonists
ADRA1 antagonists are a type of drugs that act as antagonists or blockers of the alpha-1 adrenergic receptors. Alpha-1 adrenergic receptors are a type of receptor found in various tissues in the body, including blood vessels, smooth muscles, and the central nervous system. These receptors are involved in regulating processes such as blood pressure, smooth muscle contraction, and neurotransmitter release.
ADRA1 antagonists work by binding to the alpha-1 adrenergic receptors and blocking their activation by endogenous ligands like norepinephrine. By doing so, these antagonists inhibit the effects of the sympathetic nervous system, which is responsible for the "fight or flight" response. This leads to relaxation of smooth muscles, dilation of blood vessels, and a decrease in blood pressure.
In a biomedical perspective, ADRA1 antagonists are commonly used in the treatment of conditions such as hypertension (high blood pressure), benign prostatic hyperplasia (enlarged prostate), and certain cardiovascular disorders. By blocking the alpha-1 adrenergic receptors, these drugs help to reduce blood pressure, relieve urinary symptoms associated with an enlarged prostate, and improve blood flow in certain cardiovascular conditions.
It's important to note that ADRA1 antagonists can have side effects, including dizziness, orthostatic hypotension (a drop in blood pressure upon standing up), and potential interactions with other medications. Therefore, these drugs should be used under the supervision of a healthcare professional and according to prescribed guidelines.
Drug Target R&D Trends for urapidil
ADRA1, or alpha-1 adrenergic receptors, play a crucial role in the human body. These receptors are found in various tissues and organs, including the smooth muscles of blood vessels, urinary bladder, and prostate gland. Activation of ADRA1 receptors leads to vasoconstriction, which helps regulate blood pressure. Additionally, ADRA1 receptors are involved in the contraction of smooth muscles in the urinary bladder and prostate, contributing to urinary control. Understanding the role of ADRA1 receptors is essential in the development of drugs targeting these receptors, which can be used to treat conditions such as hypertension, benign prostatic hyperplasia, and urinary incontinence.
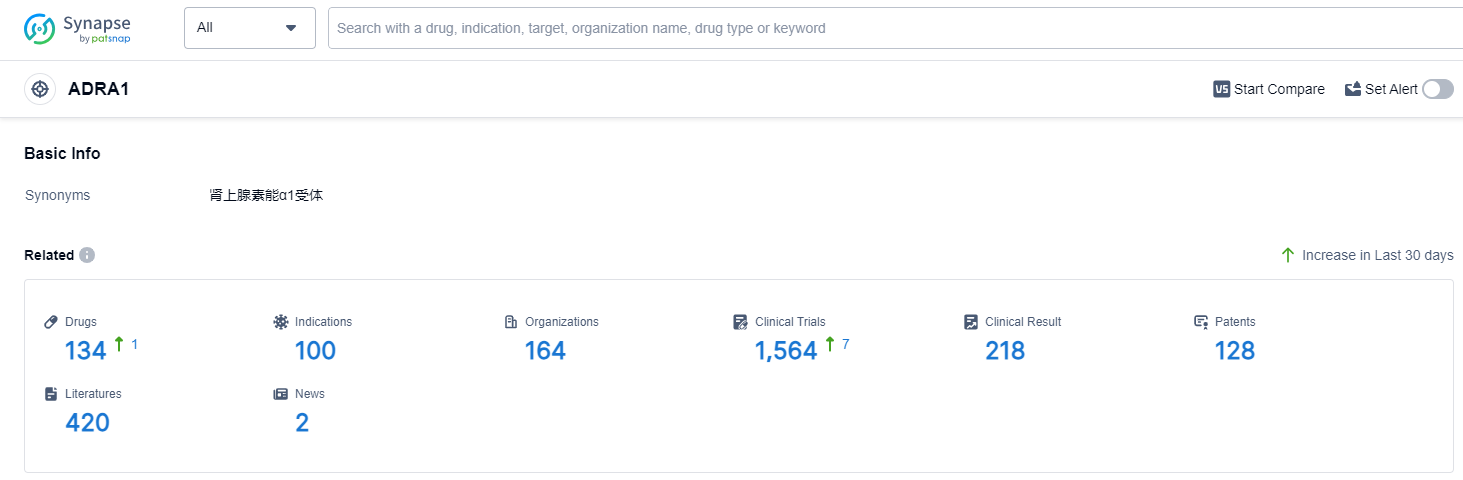
According to Patsnap Synapse, as of 13 Sep 2023, there are a total of 134 ADRA1 drugs worldwide, from 164 organizations, covering 100 indications, and conducting 1564 clinical trials.
In conclusion, the analysis of target ADRA1 in the pharmaceutical industry reveals that GSK Plc, Pfizer Inc., Sanofi, Merck & Co., Inc., and Eisai Co., Ltd. are the companies growing fastest under this target. The highest stage of development on this target is the approved phase, with small molecule drugs being the most rapidly progressing drug type. The primary indications for drugs targeting ADRA1 are hypertension, nasal obstruction, and common cold. China, the United States, Japan, and the European Union are the countries/locations developing fastest under this target, with China showing significant progress. The current competitive landscape of target ADRA1 is characterized by intense competition around small molecule drugs, primarily focused on treating hypertension, nasal obstruction, and common cold. The future development of target ADRA1 is expected to continue with a strong emphasis on R&D and expanding presence in China and other key countries/locations.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Urapidil is a small molecule drug that targets the ADRA1 receptor and has been approved for the treatment of cardiovascular diseases, urogenital diseases, and other conditions. Its active indication includes dysuria, urination disorders, and hypertension. With its global approvals, Urapidil has established itself as a reliable medication in the pharmaceutical industry, providing therapeutic benefits to patients suffering from these medical conditions.