Fate Therapeutics Announces Start of First Human Trial with FT825/ONO-8250 for HER2-positive Advanced Solid Tumors
Fate Therapeutics, Inc., an enterprise operating in the clinical-phase realm of biopharmaceuticals and focused on pioneering a novel lineup of cellular treatments for oncology and autoimmune diseases derived from induced pluripotent stem cells (iPSC), recently declared the commencement of patient recruitment for its inaugural Phase 1 clinical evaluation of FT825 / ONO-8250. This investigational candidate is a sophisticatedly engineered product of T-cells expressing chimeric antigen receptors, specifically designed to home in on and engage the human epidermal growth factor receptor 2 (HER2).
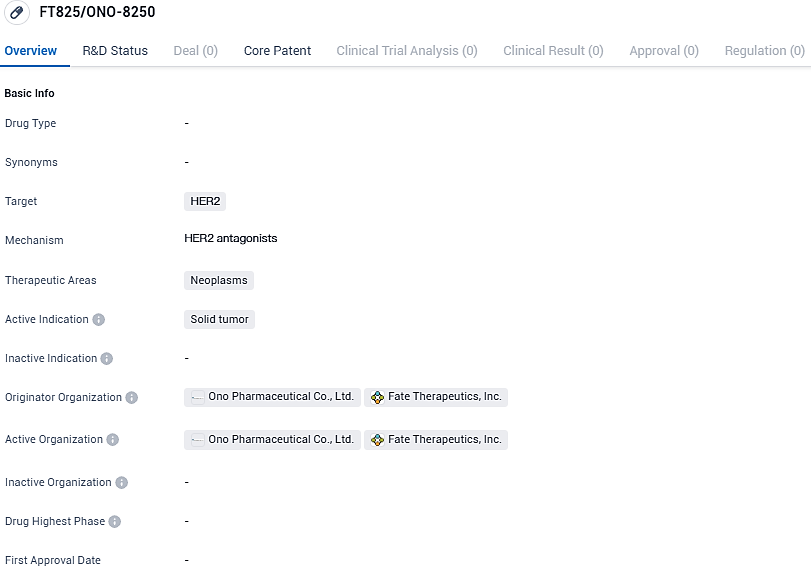
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The candidate therapeutic product, originating from iPSCs and engineered into CAR T cells, integrates a distinct domain that binds to the HER2 antigen, tailored specifically for the intricate hurdles presented by solid tumors. Currently, the initial clinical trial for FT825 / ONO-8250 progresses through a concerted effort with Ono Pharmaceutical Co., Ltd.
"Our alliance with Ono that commenced in 2018 has been deeply collaborative, as we have jointly ventured into creating CD8 alpha-beta T cells from iPSCs, and have injected innovative, synthetic regulatory elements into our iPSC-based CAR T-cell therapy platform. These enhancements aim to provide secure and potent treatment options for solid cancers by fostering cell migration, countering the immunosuppressive nature of the tumor environment, and honing in on malignant cells," remarked Scott Wolchko, who leads Fate Therapeutics as President and CEO.
Wolchko further stated, "Our laboratory analyses of FT825 / ONO-8250 signal an unmatched treatment profile against a variety of solid cancers, where the unique HER2-specific binding segment effectively distinguishes tumor cells with varying levels of HER2. It's with great anticipation that we launch the Phase 1 trial alongside Ono to explore its capabilities in benefiting patients with advanced solid tumors, a patient group facing sparse therapeutic choices."
In accordance with the collaborative and option-based contract with Ono, Fate has the responsibility to harmoniously develop and market FT825 / ONO-8250 alongside Ono within the United States and Europe, while Ono holds sole rights for the candidate's development and marketing in other international territories. As per the agreement, Fate is poised to receive payments pegged to developmental, regulatory, and commercial milestones, in addition to staggered royalties from Ono's net sales outside the American and European markets.
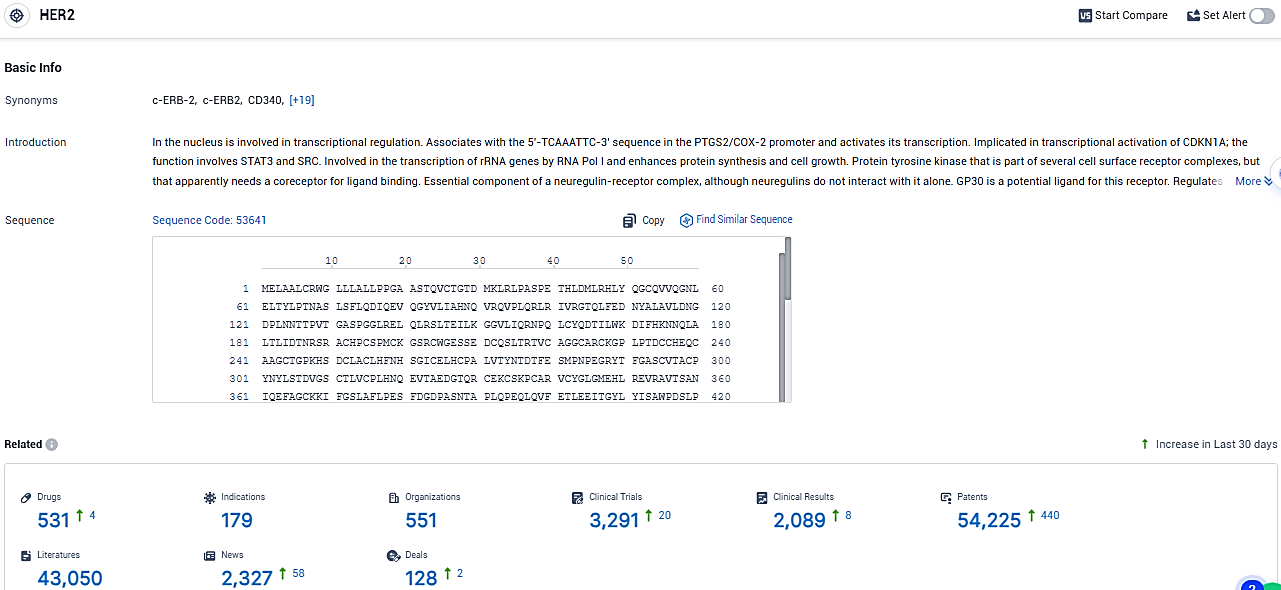
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of January 13, 2024, there are 531 investigational drugs for the HER2 tagets, including 179 indications, 551 R&D institutions involved, with related clinical trials reaching 3291, and as many as 54225 patents.
FT825/ONO-8250 is a drug that targets HER2 and is used for the treatment of neoplasms, specifically solid tumors. The drug's development highlights the significance of partnerships in the pharmaceutical industry and offers potential benefits for patients with HER2-positive cancers.