FDA Approves Atom Bioscience to Conduct Human Trials with ABP-745 for Sudden Gout Flare-ups
Atom Bioscience, a biotech firm in the clinical phase focusing on pioneering treatments for inflammation and metabolic conditions, has revealed the clearance of its Investigational New Drug submission by the U.S. Food and Drug Administration for a Phase 1 clinical study of their investigational compound, ABP-745.
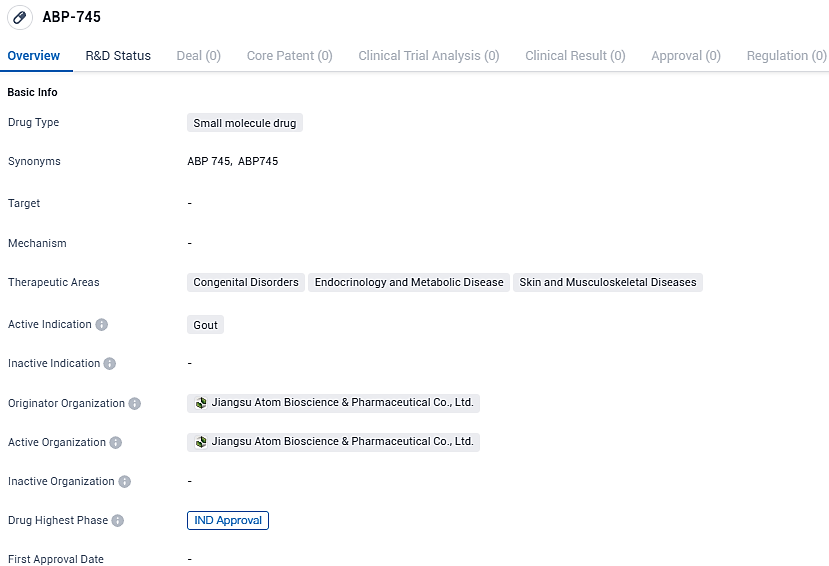
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The pharmaceutical entity known as ABP-745, a digestively absorbed medication with anti-inflammatory characteristics, is engineered to combat the occurrence of acute gout. Furthering its commitment to healthcare, Atom Biosciences has lodged a request for an IND to commence a Phase 1 trial within China's borders for ABP-745. These investigations are planned to progress simultaneously in both the United States and China.
The data from preclinical evaluations indicate a substantial diminution in the inflammatory biomarkers in test animals upon treatment with ABP-745. When juxtaposed with drugs of a similar nature, ABP-745 showcases notable advantages in both effectiveness and safety profiles. Although primarily targeted for acute gout therapy, the observed suppression of diverse inflammatory biomarkers positions ABP-745 as a potential candidate for further therapeutic uses.
Atom Biosciences' leader, Dr. William Dongfang Shi, who is the company's inception architect and presides as Chairman and CEO, remarked that the go-ahead on the ABP-745 IND represents a stride forward for the company's clinical development programs, particularly in the sphere of anti-inflammatory research. Dr. Shi anticipates the commencement of the U.S. Phase 1 trials targeting acute gout shortly, with plans to escalate investments in various inflammatory conditions to meet the pressing health needs while diversifying and enhancing Atom's product portfolio.
An acute gout episode is marked by intense, abrupt pain and inflammation in the joints, predominantly affecting the lower extremities, brought on by the accumulation of urate crystals. The crystalline formation stems from an elevated surplus of uric acid in the bloodstream, defined as hyperuricemia, with levels surpassing 7 mg/dL. This condition can give rise to chronic gout, and patients may experience recurrent acute gout assaults.
Gout, recognized as a prevalent form of inflammatory arthritis, afflicts upwards of 50 million individuals across the globe. There is an expanding demographic of patients within the United States, Europe, Asia, and Latin America. Chronic gout, without proper management, can deteriorate a patient's life quality substantially, and it correlates with increased risks of serious health issues such as renal impairment and heightened chances of sudden cardiac demise.
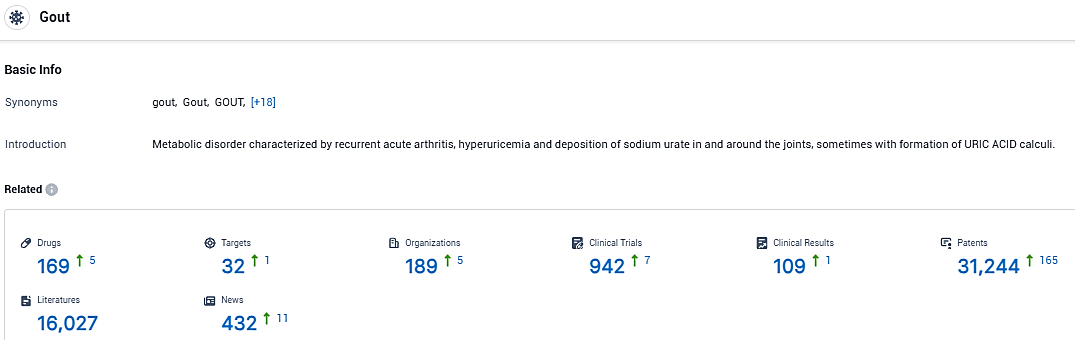
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
According to the data provided by the Synapse Database, As of January 6, 2024, there are 169 investigational drugs for the gout, including 32 targets, 189 R&D institutions involved, with related clinical trials reaching 942, and as many as 31244 patents.
ABP-745 shows promise as a potential treatment for gout, a debilitating condition that affects a significant number of individuals worldwide. Its progression to the IND Approval phase globally and IND Application phase in China indicates that it has undergone rigorous evaluation and has shown promising results in preclinical testing.