FDA Approves Ubix Therapeutics' First Clinical Trial for Oral BTK Inhibitor UBX-303-1 to Treat Resistant B-Cell Cancers
Ubix Therapeutics, Inc., a biotech firm focused on creating groundbreaking cancer treatments through the mechanism of targeted protein degradation, has disclosed that the U.S. Food and Drug Administration has granted approval to commence with the firm’s exploratory new pharmaceutical submission for UBX-303-1. This oral degradation agent is designed for the management of recurrent or resistant B-cell malignancies.
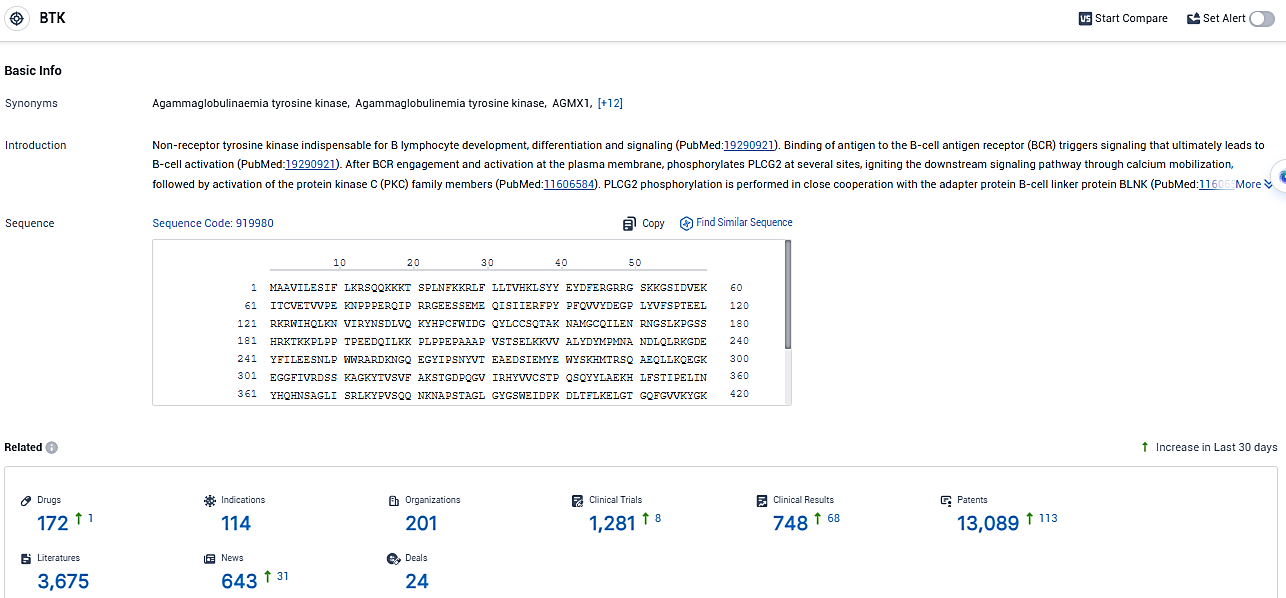
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Ubix is set to commence a preliminary phase 1 trial within the United States upon receiving authorization, with intention to branch out the research to testing locations across Europe and South Korea soon thereafter. This exploratory phase 1a/1b trial will be an open-label study, focusing on dose-escalation and expansion to ascertain the security, pharmacokinetic properties, and pharmacodynamic effects of UBX-303-1 on individuals suffering from recurring or resistant B-cell cancers. The inaugural dosing for a participant is projected for the earlier part of 2024.
UBX-303-1 is a novel, potentially superior small molecule degrader that selectively targets Bruton’s tyrosine kinase (BTK). BTK is instrumental in the pathway of B cell receptor signaling, which is essential for the proliferation and survival of cancer cells in B-cell malignancies, especially when signaling goes awry. Notably, close to 50% of patients receiving current BTK inhibitor treatments terminate their use due to severe side effects such as atrial fibrillation, hemorrhage, cardiac failure, and the evolution of drug-resistant mutations.
During preclinical tests, UBX-303-1 showed promising selectivity towards its target and potent inhibition of tumor proliferation in both cell culture and animal models. UBX-303-1 has also proven to be effective in dismantling BTK proteins in the presence of a spectrum of resistant mutations.
Ubix Therapeutics' Chief Executive Officer, BK Seo, remarked, “The green light for the Investigational New Drug (IND) application for UBX-303-1 marks a pivotal accomplishment for our enterprise and the Targeted Protein Degradation (TPD) sector. UBX-303-1 represents the inaugural TPD medication fashioned through our in-house Degraducer® technology and now moving towards clinical testing. We are optimistic that UBX-303-1 may introduce novel therapeutic paths for patients grappling with recurring or resistant B-cell cancers. As Ubix advances its TPD project line-up, our pledge to innovation is unwavering in our mission to deliver groundbreaking treatments to those in medical need.”
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of January 6, 2024, there are 172 investigational drugs for the BTK target, including 114 indications, 201 R&D institutions involved, with related clinical trials reaching 1281, and as many as 13089 patents.
UBX-303-1 is a PROTACs drug that targets BTK and has potential applications in the treatment of hematologic neoplasms. It is currently in the preclinical phase of development. Further research and clinical trials will be needed to determine the safety and efficacy of UBX-303-1 in treating hematologic neoplasms.