FDA Approves BIMZELX® as First IL-17A/IL-17F Blocker for Hidradenitis Suppurativa
UCB, an international biopharmaceutical firm, has announced that BIMZELX® (bimekizumab-bkzx) has received approval from the U.S. Food and Drug Administration (FDA) for use in treating adults suffering from moderate to severe hidradenitis suppurativa (HS). This medication, bimekizumab-bkzx, is the first of its kind to specifically target and inhibit both interleukin 17F (IL-17F) and interleukin 17A (IL-17A).
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"The recent endorsement of BIMZELX for treating moderate to severe hidradenitis suppurativa is a significant advancement, considering the considerable unmet medical needs and the limited treatment choices currently available," stated Alexa B. Kimball, MD, MPH, an investigator and the principal author of the studies from Beth Israel Deaconess Medical Center and a Professor of Dermatology at Harvard Medical School in Boston, MA, U.S. "In the Phase 3 clinical trials, patients receiving bimekizumab-bkzx experienced profound and lasting clinical improvements lasting up to 48 weeks."
Hidradenitis suppurativa is a chronic inflammatory skin condition that reoccurs, is painful, and can be debilitating. Key symptoms include the presence of nodules, abscesses, and fistulas that discharge pus, typically found in areas such as the armpits, groin, and buttocks. Individuals with HS frequently endure flare-ups and significant pain, which can substantially affect their quality of life.
"We aim to create a future where individuals with hidradenitis suppurativa can live without stigma and receive effective treatment," remarked Brindley Brooks, Founder and Executive Director of HS Connect, U.S. "The approval of bimekizumab-bkzx marks an encouraging moment for the hidradenitis suppurativa community, providing new treatment options for those in the U.S. suffering from moderate to severe cases."
The approval is backed by findings from two Phase 3 trials, BE HEARD I and BE HEARD II, which assessed the safety and effectiveness of bimekizumab-bkzx in adults with moderate to severe HS. The studies indicated that a greater percentage of patients treated with bimekizumab-bkzx, compared to those receiving a placebo, realized a 50% or greater reduction in HS signs and symptoms by Week 16, as indicated by HiSCR50, the main outcome measure in both studies. Additionally, bimekizumab-bkzx treatment led to meaningful improvements in the key secondary endpoint, HiSCR75, compared to placebo at Week 16, with clinical benefits maintaining up to Week 48. The safety profile of bimekizumab-bkzx was consistent with previous trial data across various indications, showing no novel safety concerns. Comprehensive results from BE HEARD I and II have been published in The Lancet.
"We are delighted that with this achievement, BIMZELX is now FDA-approved for adults with moderate to severe hidradenitis suppurativa, a chronic and painful condition affecting roughly one in every 100 individuals. This marks the fifth patient group that could benefit from BIMZELX in the U.S., representing a significant advancement in our efforts to reduce the global burden of immune-mediated inflammatory diseases," said Emmanuel Caeymaex, Executive Vice President, Patient Impact Lead and Chief Commercial Officer at UCB. "This achievement highlights our dedication to addressing the unmet needs in hidradenitis suppurativa and other immunological disorders by providing innovative treatments and enhancing care standards."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
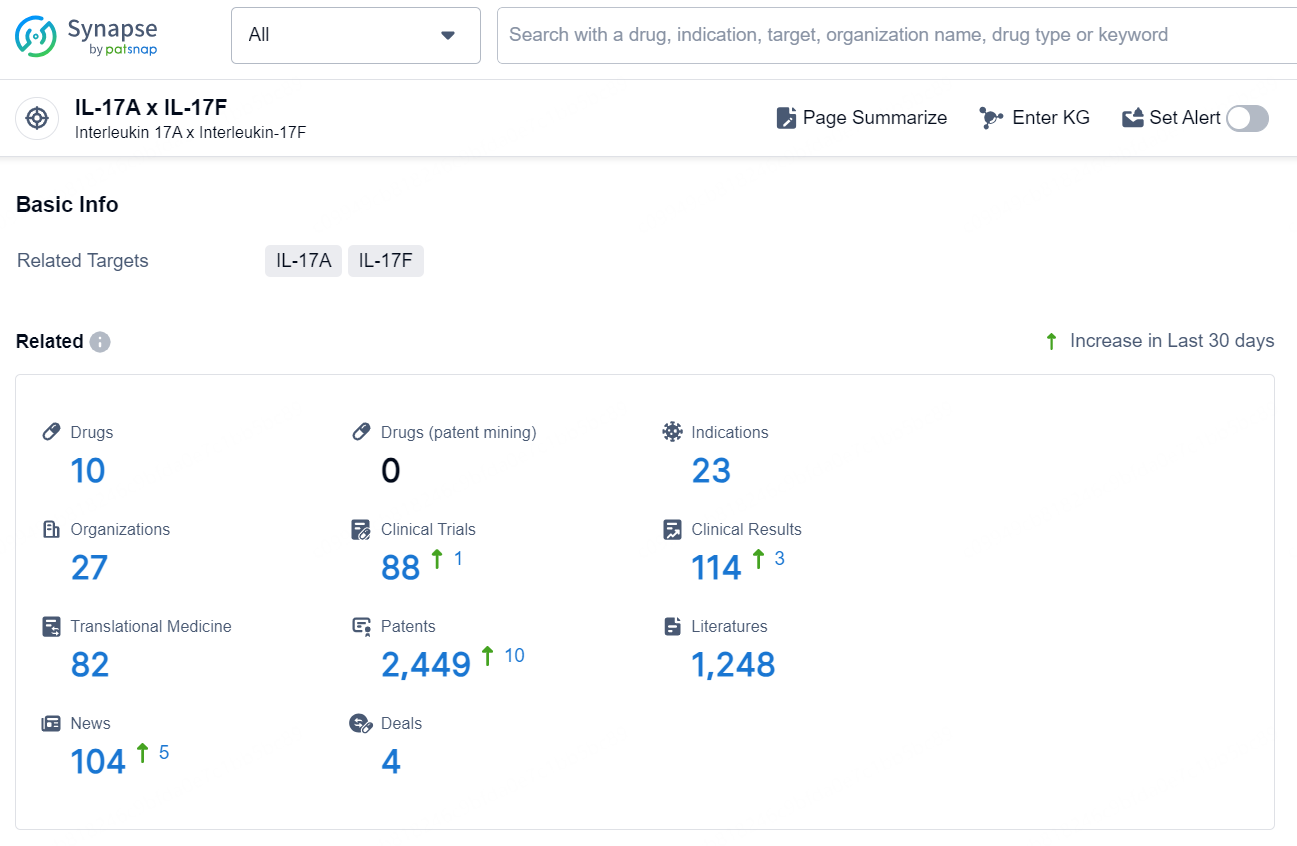
According to the data provided by the Synapse Database, As of November 25, 2024, there are 10 investigational drugs for the IL-17A x IL-17F target, including 23 indications, 27 R&D institutions involved, with related clinical trials reaching 88, and as many as 2449 patents.
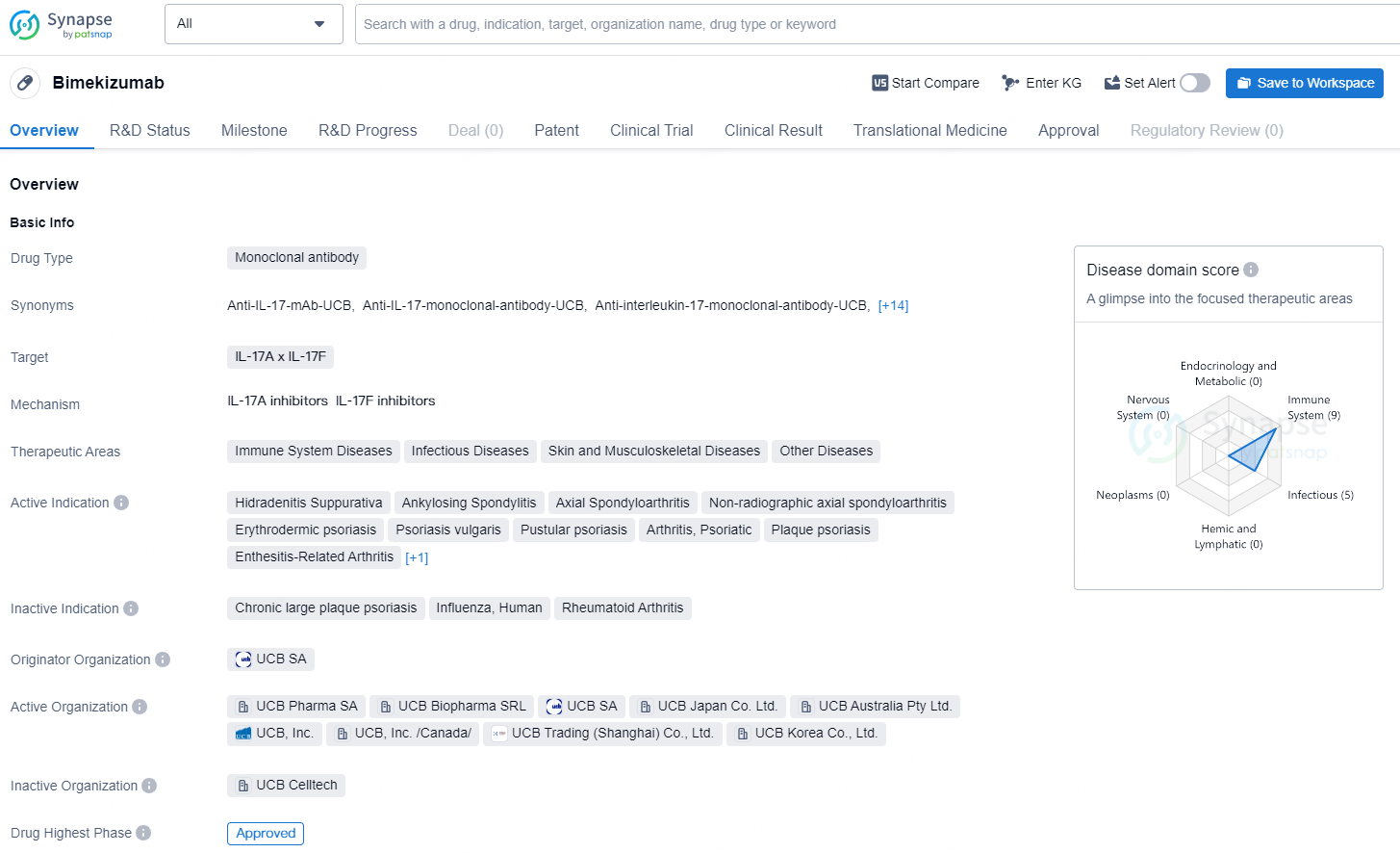
Bimekizumab is a monoclonal antibody drug that targets IL-17A and IL-17F and is indicated for a range of immune system, infectious, skin, musculoskeletal, and other diseases. Its active indications include Hidradenitis Suppurativa, Ankylosing Spondylitis, Axial Spondyloarthritis, Non-radiographic axial spondyloarthritis, Erythrodermic psoriasis, Psoriasis vulgaris, Pustular psoriasis, Arthritis, Psoriatic, Plaque psoriasis, Enthesitis-Related Arthritis, and Juvenile Idiopathic Arthritis.