Merck's Phase 3 Trial for Subcutaneous Pembrolizumab Achieves Primary Goals
Merck (NYSE: MRK), referred to as MSD in regions outside the United States and Canada, has reported encouraging topline findings from the pivotal Phase 3 MK-3475A-D77 trial. This study is assessing whether the subcutaneous delivery of pembrolizumab, Merck’s PD-1 inhibitor also known as KEYTRUDA® in its IV form, combined with berahyaluronidase alfa, a hyaluronidase variant produced by Alteogen Inc. (MK-3475A; subcutaneous pembrolizumab), is noninferior to the intravenous administration of KEYTRUDA alongside chemotherapy. This trial targets first-line treatment for adult patients diagnosed with metastatic non-small cell lung cancer (NSCLC).
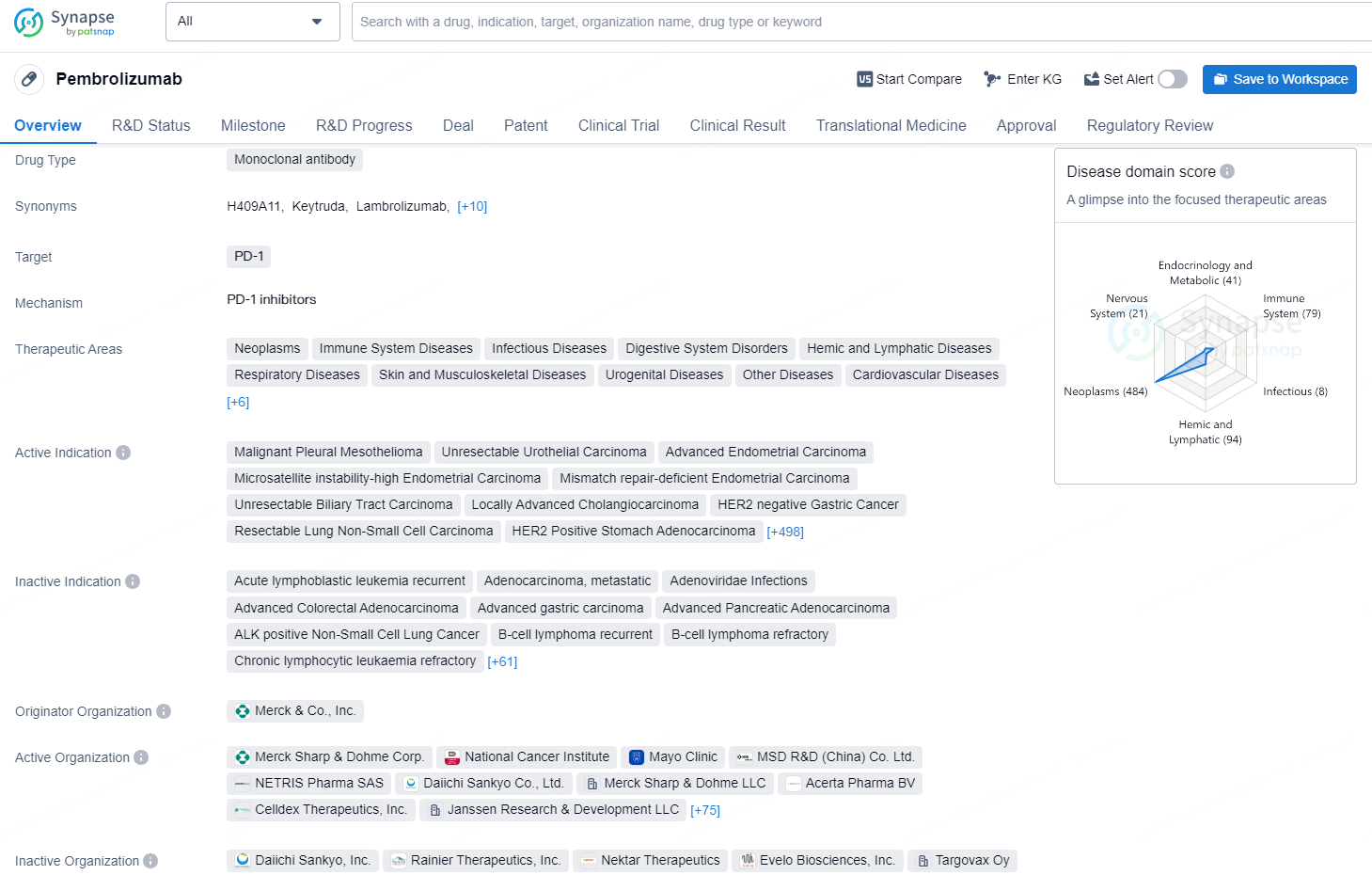
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The Phase 3 study successfully achieved its dual primary pharmacokinetic (PK) objectives. In particular, the administration of subcutaneous pembrolizumab every six weeks along with chemotherapy showed noninferiority in terms of Area Under the Curve (AUC) exposure during the initial dosing cycle and the trough concentration (Ctrough) at steady state when compared to intravenous KEYTRUDA given every six weeks with chemotherapy. Furthermore, secondary endpoints concerning efficacy and safety were generally aligned between subcutaneous pembrolizumab with chemotherapy and intravenous KEYTRUDA with chemotherapy. These findings, along with other ongoing analyses, are set to be presented at an upcoming medical conference and communicated to regulatory bodies globally.
"KEYTRUDA has significantly changed the treatment landscape for some of the most severe cancers, and we are committed to exploring further innovations that could benefit patients," stated Dr. Marjorie Green, senior vice president and head of oncology at Merck Research Laboratories. "It is very promising to see favorable Phase 3 results for this fixed-dose regimen of subcutaneous pembrolizumab, which can be administered in about 2-3 minutes on average. This approach has the potential to enhance patient experience and improve access for both patients and healthcare providers when compared to intravenous delivery. We aim to discuss these results with regulatory agencies worldwide at the earliest opportunity."
In addition to the MK-3475A-D77 Phase 3 trial, Merck's clinical development program for subcutaneous pembrolizumab also includes the MK-3475A-F84 Phase 3 trial, which investigates subcutaneous pembrolizumab monotherapy against IV KEYTRUDA monotherapy in first-line treatment for metastatic NSCLC patients with high PD-L1 expression (tumor proportion score [TPS] ≥50%). Additionally, the Phase 2 MK-3475A-F65 trial is examining subcutaneous pembrolizumab on its own in cases of relapsed or refractory classical Hodgkin lymphoma and relapsed or refractory primary mediastinal large B-cell lymphoma. Furthermore, Merck is conducting a Phase 2 patient preference study, MK-3475A-F11, which assesses participant preferences between subcutaneous pembrolizumab and IV KEYTRUDA.
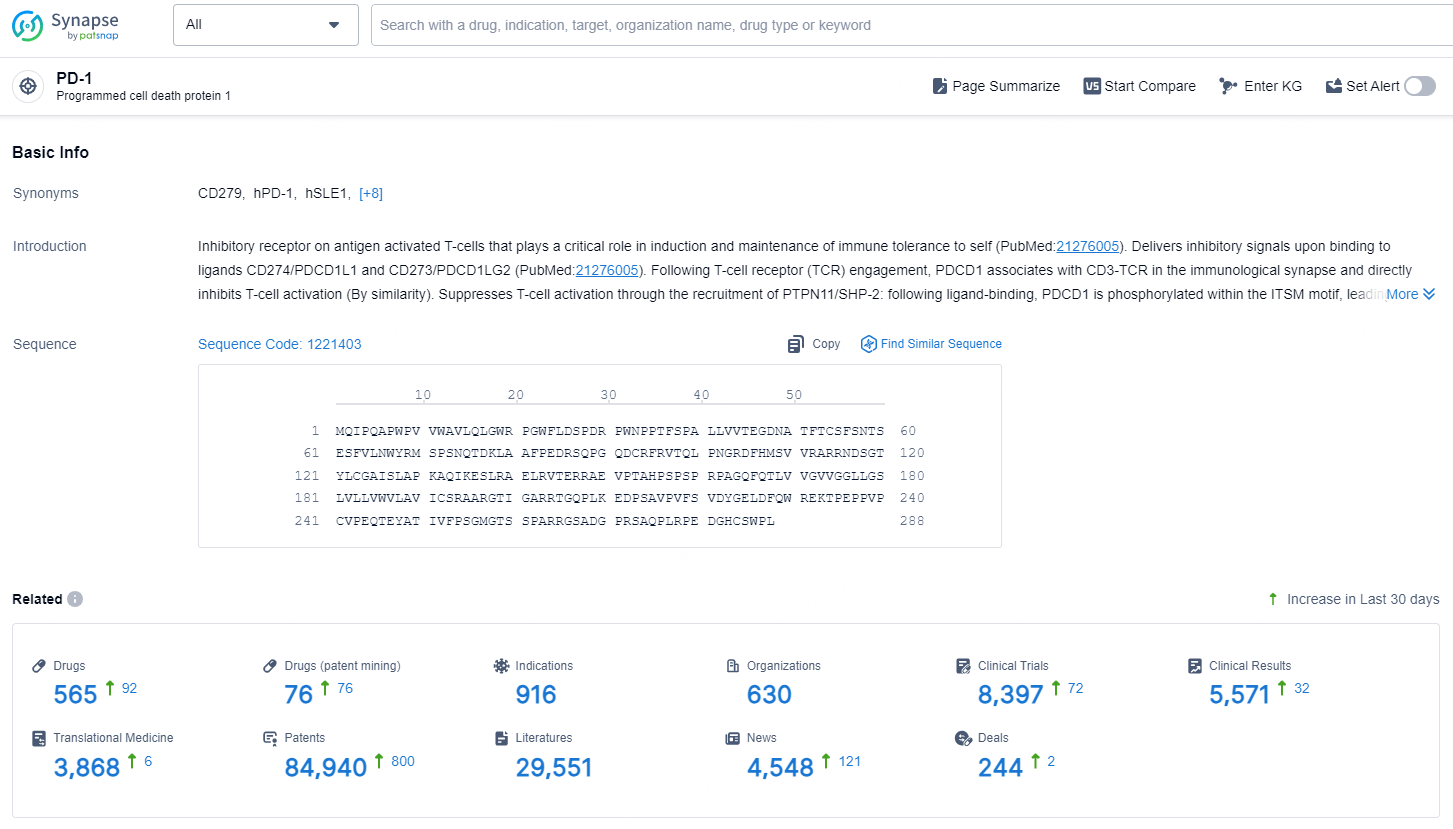
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of November 20, 2024, there are 565 investigational drugs for the PD-1 target, including 916 indications, 630 R&D institutions involved, with related clinical trials reaching 8397, and as many as 84940 patents.
Pembrolizumab is a monoclonal antibody that targets the PD-1 receptor and is approved for various therapeutic areas including neoplasms, immune system diseases, infectious diseases, digestive system disorders, and many others. The drug has a wide range of active indications, including several types of carcinoma, lymphoma, and other malignancies. It was first approved in the United States in September 2014, and it has also received approvals in China.