FDA Approves Citius's LYMPHIR™ BLA Revision for Adult Cutaneous T-Cell Lymphoma
Citius Pharmaceuticals, Inc., has disclosed that the FDA has given the green light to the company's revised Biologics License Application for its product LYMPHIR™ (denileukin diftitox). This innovative immunotherapy, which harnesses the power of IL-2, is designed to combat cutaneous T-cell lymphoma in individuals who have not responded to or have experienced a relapse following at least one prior systemic treatment. The FDA has scheduled a target date for its decision-making process, as per the Prescription Drug User Fee Act (PDUFA), which is set for the 13th of August, 2024.
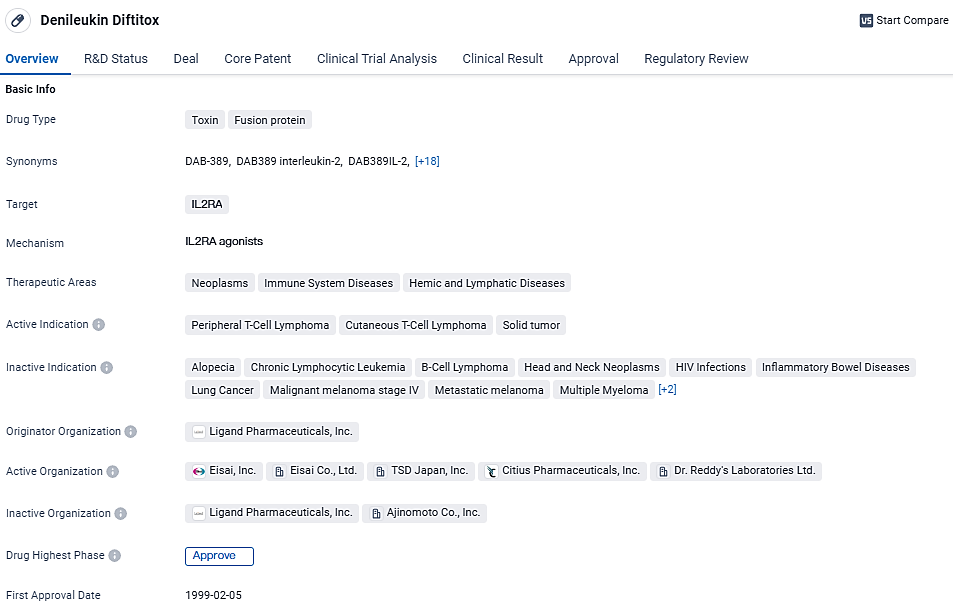
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"The resubmission of the Biologics License Application (BLA) has been acknowledged as complete, addressing the extensive product testing and added measures underscored by the FDA in the CRL from July 2023. The correspondence did not raise any issues regarding safety or the efficacy of the product, and our confidence in the comprehensive clinical data presented in the initial BLA filing stands firm," declared Leonard Mazur, the Chairman and CEO of Citius Pharmaceuticals.
Mazur continued, "There's a pressing need for more effective treatments for patients experiencing the recurrence or resistance of CTCL since existing treatments do not offer a cure. We appreciate the FDA's commitment to fostering the development of drugs for rare diseases, and we're committed to enhancing the treatment landscape for individuals with cutaneous T-cell lymphoma. We eagerly await the FDA's verdict on our application and the potential for LYMPHIR to improve outcomes for those affected by CTCL."
The BLA is buttressed by a significant Phase 3 research study. This resubmission came about after constructive conversations with the FDA, spurred by a Complete Response Letter issued on the 28th of July, 2023. Citius is confident it has remedied the concerns regarding product testing and the manufacturing process improvements mentioned in the correspondence.
LYMPHIR represents an innovative treatment, devised as a recombinant fusion protein that merges the interleukin-2 (IL-2) receptor binding domain with fragments of diphtheria toxin. This therapeutic specifically targets IL-2 receptors on cell membranes, leading to the cellular uptake of the toxin fragments which then disrupt protein manufacturing within those cells.
Previously, in 2011 and 2013, the FDA conferred orphan drug status on LYMPHIR for the indications of PTCL and CTCL. In 2021, a similar agent, denileukin diftitox, gained market authorization in Japan for treating CTCL and peripheral T-cell lymphoma. That same year, Citius obtained exclusive licensing rights to advance and market LYMPHIR in all territories apart from Japan and certain regions in Asia.
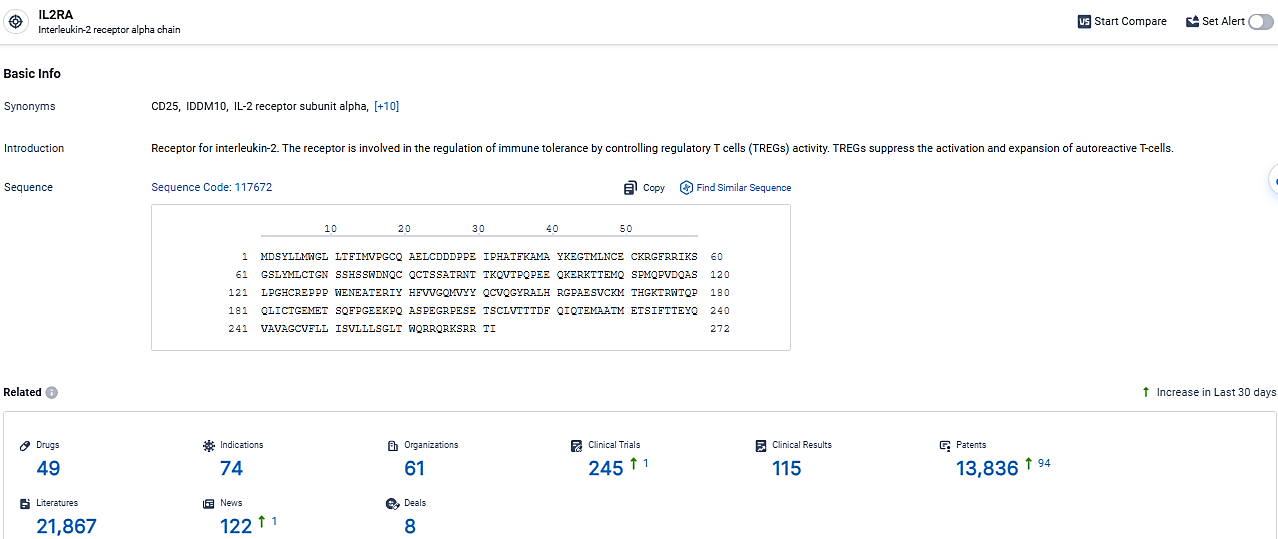
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of March 19 2024, there are 49 investigational drugs for the IL2RA target, including 74 indications, 61 R&D institutions involved, with related clinical trials reaching 245, and as many as 13836 patents.
Denileukin Diftitox targets IL2RA and is approved for the treatment of peripheral T-cell lymphoma, cutaneous T-cell lymphoma, and solid tumors. Its first approval was granted in the United States in 1999, and it received accelerated approval and orphan drug status. This drug represents an important advancement in the field of biomedicine, providing a new treatment option for patients with these specific conditions.