FDA Approves Idorsia's TRYVIO, First Endothelin Antagonist for Resistant Hypertension
Idorsia Ltd has disclosed that the American regulatory authority, the FDA, has granted official sanction for the use of TRYVIO™ (aprocitentan) as an adjunct therapy for managing elevated blood pressure. Specifically, it is indicated for adults whose hypertension is not sufficiently managed with their current medicinal regimen. This approval allows for the utilization of TRYVIO™ alongside various other antihypertensive medications to enhance blood pressure control.
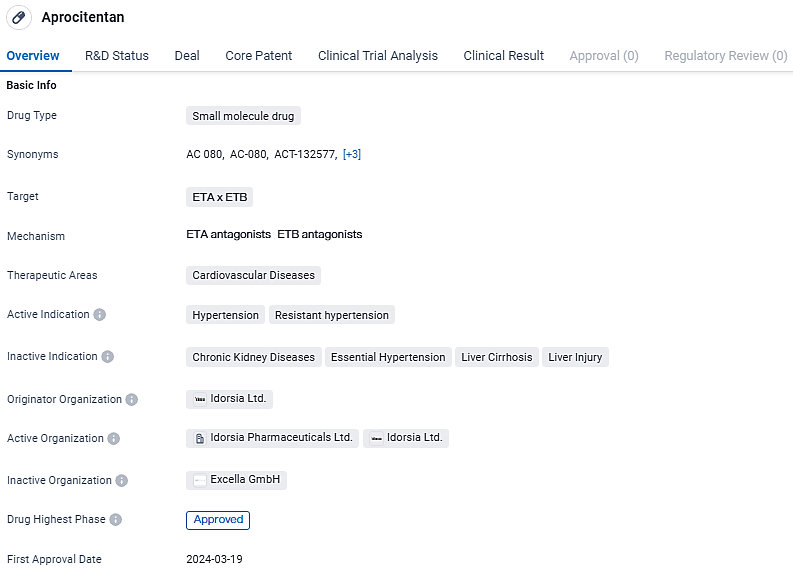
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Reducing hypertension is linked to decreased chances of both non-lethal and lethal cardiovascular incidents, most notably myocardial infarctions and cerebral strokes. The advised intake of TRYVIO comprises a daily 12.5 mg dose administered orally, which can be taken with meals or on an empty stomach.
Speaking on behalf of Idorsia, Dr. Jean-Paul Clozel, its CEO, remarked: "A significant segment of the American population struggles with inadequately managed hypertension despite the availability of current medications. This situation contributes to a substantial number of events linked to heart and brain circulatory problems. In response, Idorsia has been developing aprocitentan, a drug that antagonizes endothelin receptors, designed to assist in managing such patients."
TRYVIO (aprocitentan) operates as an antagonist to endothelin receptors, obstructing the interaction of endothelin (ET)-1 with both ETA and ETB receptors. The impact of ET-1 aligns closely with hypertension pathology, and it is a key instigator in generating aldosterone.
Before TRYVIO's sanctioning, ET pathway targeting was not an aspect of systemic hypertension treatments; the prevalent medications modulate water and salt, counteract the renin–angiotensin–aldosterone system, limit the inflow of extracellular calcium, exhibit sympatholytic action, or provide unselective vasodilation.
Investigations of TRYVIO as a sole treatment involved a Phase 2 trial with hypertensive individuals, and as a supplementary treatment in a Phase 3 trial named PRECISION, involving patients diagnosed with resistant hypertension. In the PRECISION trial, aprocitentan was generally well-received and proved more effective than a placebo in reducing hypertension at the 4-week mark, maintaining its effectiveness up to week 40.
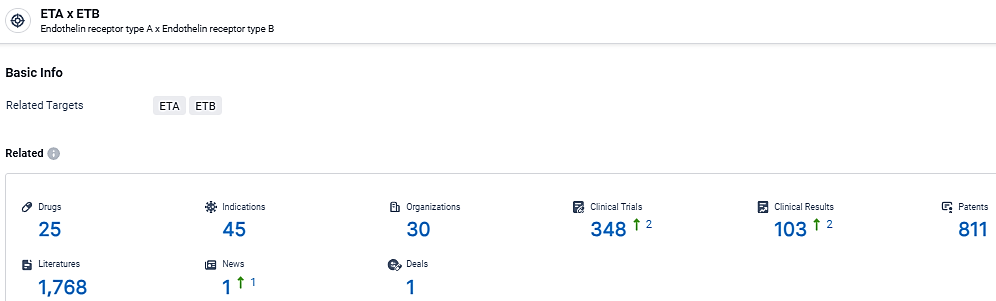
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of March 21 2024, there are 25 investigational drugs for the ETA/ETB target, including 45 indications, 30 R&D institutions involved, with related clinical trials reaching 348, and as many as 811 patents.
Aprocitentan is a small molecule drug developed for the treatment of hypertension and resistant hypertension. With its approval in the United States and ongoing Phase 3 trials in China, Aprocitentan shows promise as a potential therapeutic option for patients suffering from cardiovascular diseases.