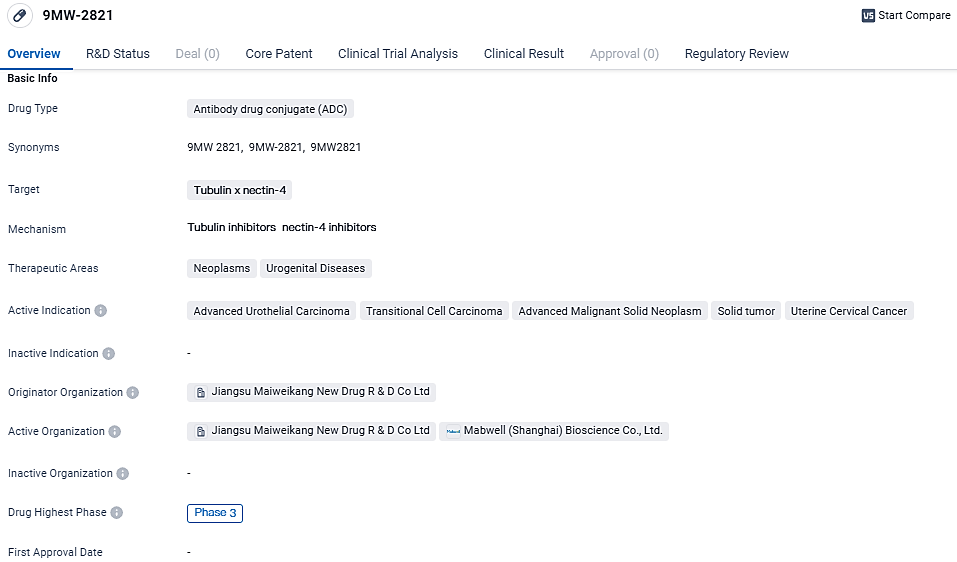
Mabwell's Novel ADC Targets Nectin-4, Shows Promise in Cervical Cancer Treatment
Mabwell, a pioneering company in the biopharmaceutical field that operates a comprehensive industry chain, showcased the results from its clinical trials for their drug 9MW2821, aimed at treating individuals with cervical cancer. This significant data was unveiled in the form of a dedicated plenary speech at the annual conference of the Society of Gynecologic Oncology.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Clinical research indicates promising results regarding the effectiveness and safety profile of the therapeutic agent 9MW2821 when administered to individuals with cervical cancer. This agent is anticipated to pave the way for innovative treatment modalities aimed at addressing recurring or metastatic forms of cervical cancer, thereby fulfilling numerous presently unaddressed clinical requirements. Of all the medications targeting the same mechanism globally, 9MW2821 is at the forefront, with reported clinical outcomes specifically for cervical cancer.
Analysis of a Nectin-4-Specific Antibody-Drug Conjugate's Clinical Trial for Recurrent or Metastatic Cervical Cancer Treatment
Under the guidance of Professor Zhang Jian from Fudan University Shanghai Cancer Center, along with Professor Yang Huijuan who represented the study group, a comprehensive description of this phase I/II, multicentric, and non-blinded clinical trial was delivered at a conference. The findings earned substantial accolades from specialists attending the event. Continued analysis of these findings could potentially introduce additional therapeutic avenues for individuals enduring recurrent or metastatic cervical cancer.
Mabwell, employing its ADC technology and automated high-capacity hybridoma antibody discovery system, has crafted 9MW2821 as a uniquely site-specific, pioneering ADC that zeroes in on Nectin-4. This molecule stands as the foremost in its category, being investigated clinically amongst its Chinese counterparts. Various clinical evaluations regarding 9MW2821 are taking place in China, focusing on its safety, tolerability, pharmacokinetics, and clinical benefits in patients with diverse advanced malignant tumors.
A phase III solo treatment trial with 9MW2821 has been launched, focusing on patients with advanced local or metastatic urothelial carcinoma who have prior exposure to platinum-containing chemotherapy and PD-1 inhibitory drugs. An initial patient has been registered in the phase I/II trials, which examine the combined application of 9MW2821 and PD-1 inhibitors.
Further patient recruitment and assessments for the phase II esophageal carcinoma studies are progressing, with preparations to hasten the phase III trial discussions. In February 2024, 9MW2821 received the Fast Track designation from the United States Food and Drug Administration for managing advanced, recurrent, or metastatic esophageal squamous cell carcinoma.
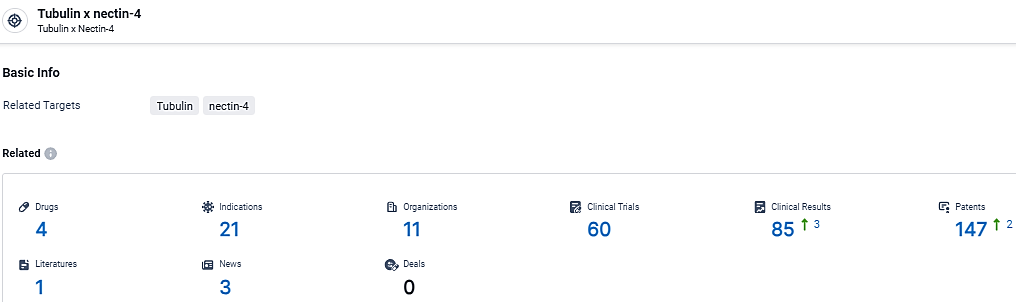
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of March 21, 2024, there are 4 investigational drugs for the Tubulin and nectin-4 target, including 21 indications, 11 R&D institutions involved, with related clinical trials reaching 60, and as many as 147 patents.
9MW-2821 shows promise as a potential treatment for various types of neoplasms and urogenital diseases. Its advancement to Phase 3 trials suggests that it has demonstrated positive results in earlier stages of development. The Fast Track designation further highlights the drug's potential to address unmet medical needs in these therapeutic areas. However, further clinical trials and regulatory approvals are required to determine its safety and efficacy before it can be made available to patients.