FDA Approves Merus' Zeno for Expedited NRG1+ Lung and Pancreatic Cancer Treatment
Merus N.V., an enterprise at the clinical development phase focusing on oncology, and specializing in the creation of innovative, full-length multispecific antibodies, has announced the acceptance of a Biologics License Application by the U.S. Food and Drug Administration for priority review. This application pertains to the bispecific antibody zenocutuzumab (Zeno), aimed at treating patients with neuregulin 1 fusion non-small cell lung cancer and NRG1+ pancreatic cancer.
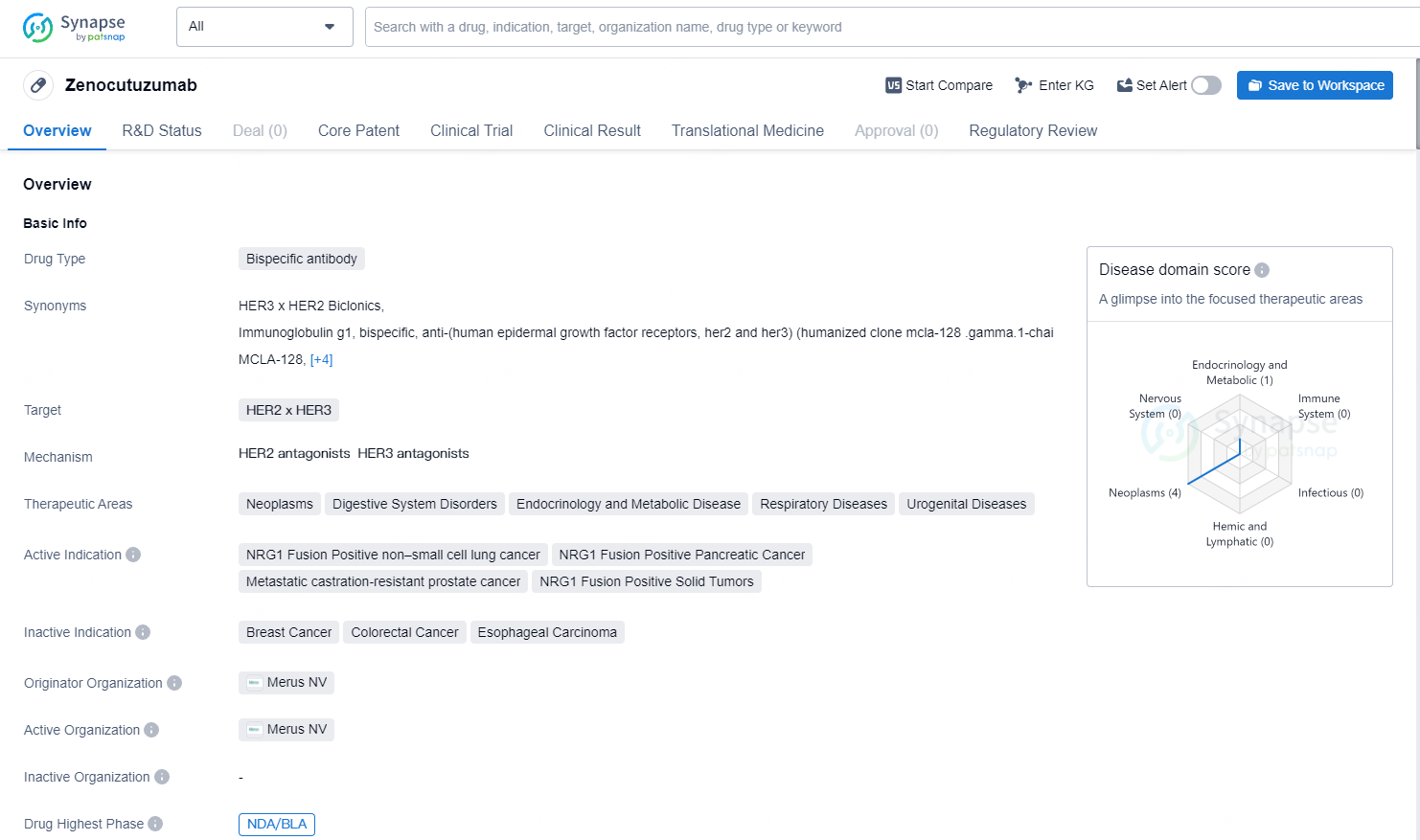
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Dr. Andrew Joe, the Chief Medical Officer at Merus, noted, "Our first BLA submission to the FDA marks a significant milestone for Merus and presents a promising treatment avenue for patients suffering from NRG1+ cancer, a malignancy characterized by a dire prognosis and a significant lack of effective treatments."
“Zenocutuzumab could become the premier exclusive treatment tailored for NRG1+ cancer affecting the lungs and pancreas, potentially offering significant advancements over existing treatment methods,” continued Dr. Joe.
The submitted BLA encompasses extensive clinical evidence, including outcomes from the phase 1/2 eNRGy study, which evaluates the efficacy and safety of Zenocutuzumab as a standalone treatment for NRG1+ NSCLC, PDAC, and various other solid tumors. The findings from this trial, an open-label study, were initially shared at the 2021 American Society of Clinical Oncology Annual Meeting and have since been updated during the 2022 ASCO meeting and the 2023 European Society for Medical Oncology Congress.
Zeno has received Breakthrough Therapy Designation from the FDA for its use in treating advanced, inoperable, or metastatic NRG1+ pancreatic cancer patients who have shown disease progression following standard systemic treatment or for whom no satisfactory alternatives exist. The FDA also awarded Breakthrough Therapy Designation for Zeno in managing sophisticated, inoperable, or metastatic NRG1+ NSCLC under similar conditions.
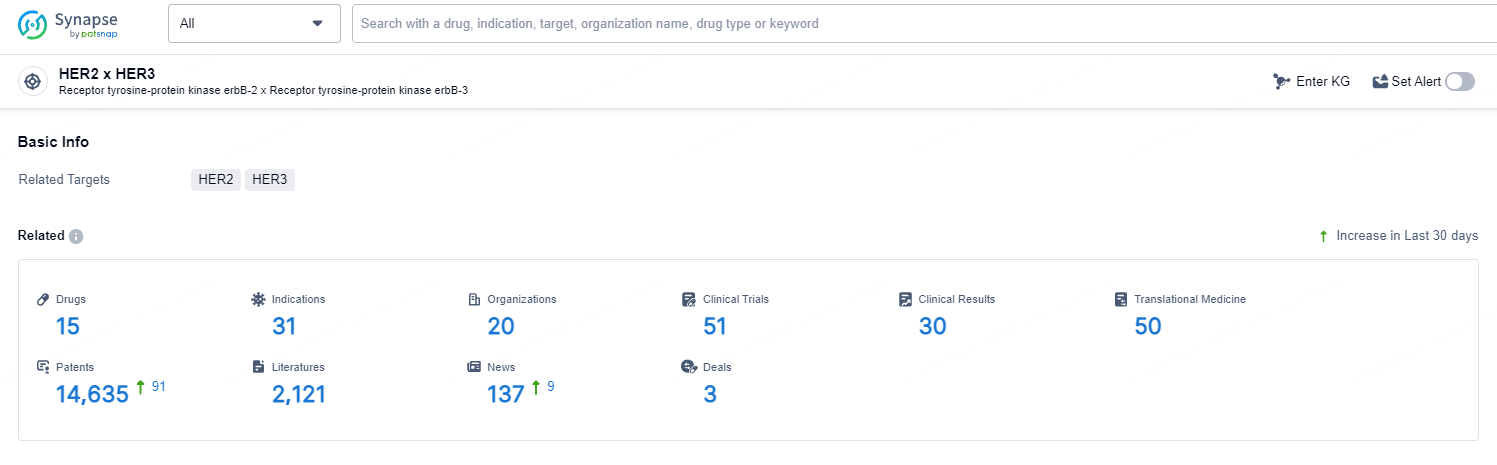
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of May 11, 2024, there are 15 investigational drugs for the HER2 and HER3 targets, including 31 indications, 20 R&D institutions involved, with related clinical trials reaching 52, and as many as 14635 patents.
Zenocutuzumab appears to be a promising drug with a broad therapeutic potential in various disease areas. Its bispecific antibody nature and targeting of HER2 x HER3 receptors suggest a novel mechanism of action that may provide unique benefits for patients. The drug's highest phase of development globally and its regulatory designations further highlight its potential as a significant advancement in the field of biomedicine. However, further clinical trials and regulatory approvals are required to fully assess its safety and efficacy.