FDA Approves Novartis' Kisqali® for Early-Stage HR+/HER2- Breast Cancer Recurrence Reduction
Novartis revealed that the FDA has given the green light to Kisqali® (ribociclib), to be used in conjunction with an aromatase inhibitor (AI), for the adjuvant therapy of patients with hormone receptor-positive/human epidermal growth factor receptor 2-negative (HR+/HER2-) stage II and III early breast cancer (EBC), who have a high probability of cancer returning. This approval also encompasses patients with node-negative (N0) disease.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The authorization is grounded on findings from the pivotal Phase III NATALEE study, which revealed a substantial and clinically valuable 25.1% (HR=0.749; 95% CI: 0.628, 0.892; P=0.0006) decrease in the risk of disease recurrence among a wide spectrum of HR+/HER2- stage II and III EBC patients treated with adjuvant Kisqali plus endocrine therapy (ET) as opposed to ET alone, encompassing those with high-risk N0 disease. The invasive disease-free survival (iDFS) advantage was consistently noted in all patient subgroups.
“The FDA's approval of Kisqali for this early breast cancer group, including those with N0 disease, marks a crucial advance in enhancing our treatment strategies,” remarked Dennis J. Slamon, M.D., Director of Clinical/Translational Research at UCLA Jonsson Comprehensive Cancer Center and Chairman of the Board of Translational Research In Oncology (TRIO), lead investigator of the NATALEE trial. “This approval enables us to provide a CDK4/6 inhibitor as a substantial component for a wider range of patients, effectively reducing their risk of cancer recurrence when combined with endocrine therapy.”
In the context of EBC, Kisqali is administered with or without food as a daily oral dose of 400 mg (two 200 mg tablets) for three weeks, followed by one week of no treatment, alongside four weeks of any AI. Patients are advised to continue Kisqali for three years. The NATALEE study demonstrated that the safety profile of Kisqali at the 400 mg dose was generally well-tolerated, with discontinuations primarily resulting from asymptomatic laboratory findings. Adverse events (AEs) of special concern in the Kisqali + ET arm of the NATALEE trial include (all Grades, and Grades 3/4, respectively): neutropenia (62.5%, 44.3%), liver-related AEs (26.4%, 8.6%), QT interval prolongation (5.3%, 1.0%), and interstitial lung disease/pneumonitis (1.5%, 0.0%).
A refreshed analysis from the NATALEE study, recently showcased at the European Society for Medical Oncology (ESMO) Congress 2024, substantiates the data evaluated by the FDA. Findings indicated an enhanced benefit beyond the three-year treatment duration and a 28.5% reduction in the risk of recurrence (HR=0.715; CI 95% 0.609-0.840; P<0.0001) compared to ET alone, among patients with stage II and III HR+/HER2- EBC7. Novartis will continue to assess NATALEE patients for extended-term outcomes, including overall survival.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
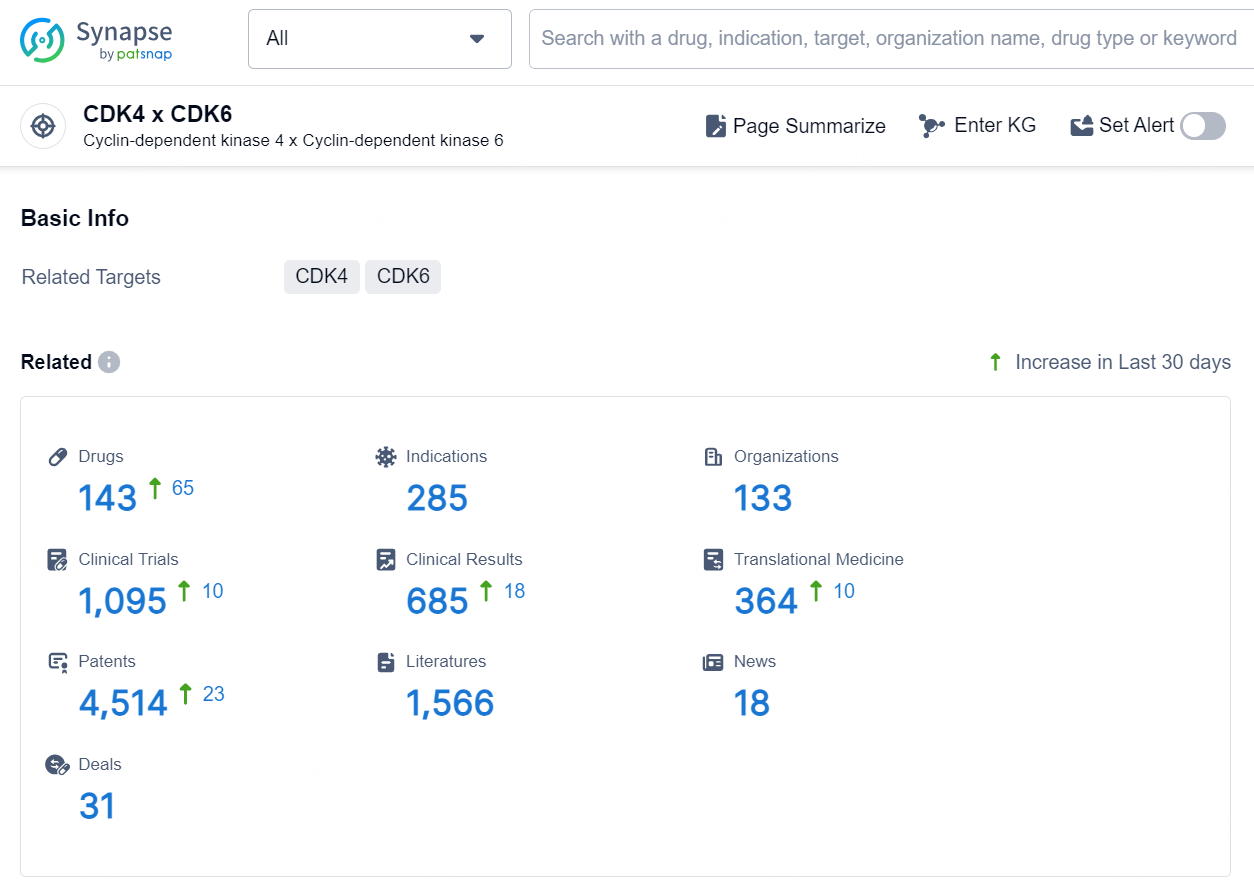
According to the data provided by the Synapse Database, As of September 18, 2024, there are 143 investigational drug for the CDK4 and CDK6 targets, including 285 indications, 133 R&D institutions involved, with related clinical trials reaching 1095, and as many as 4514 patents.
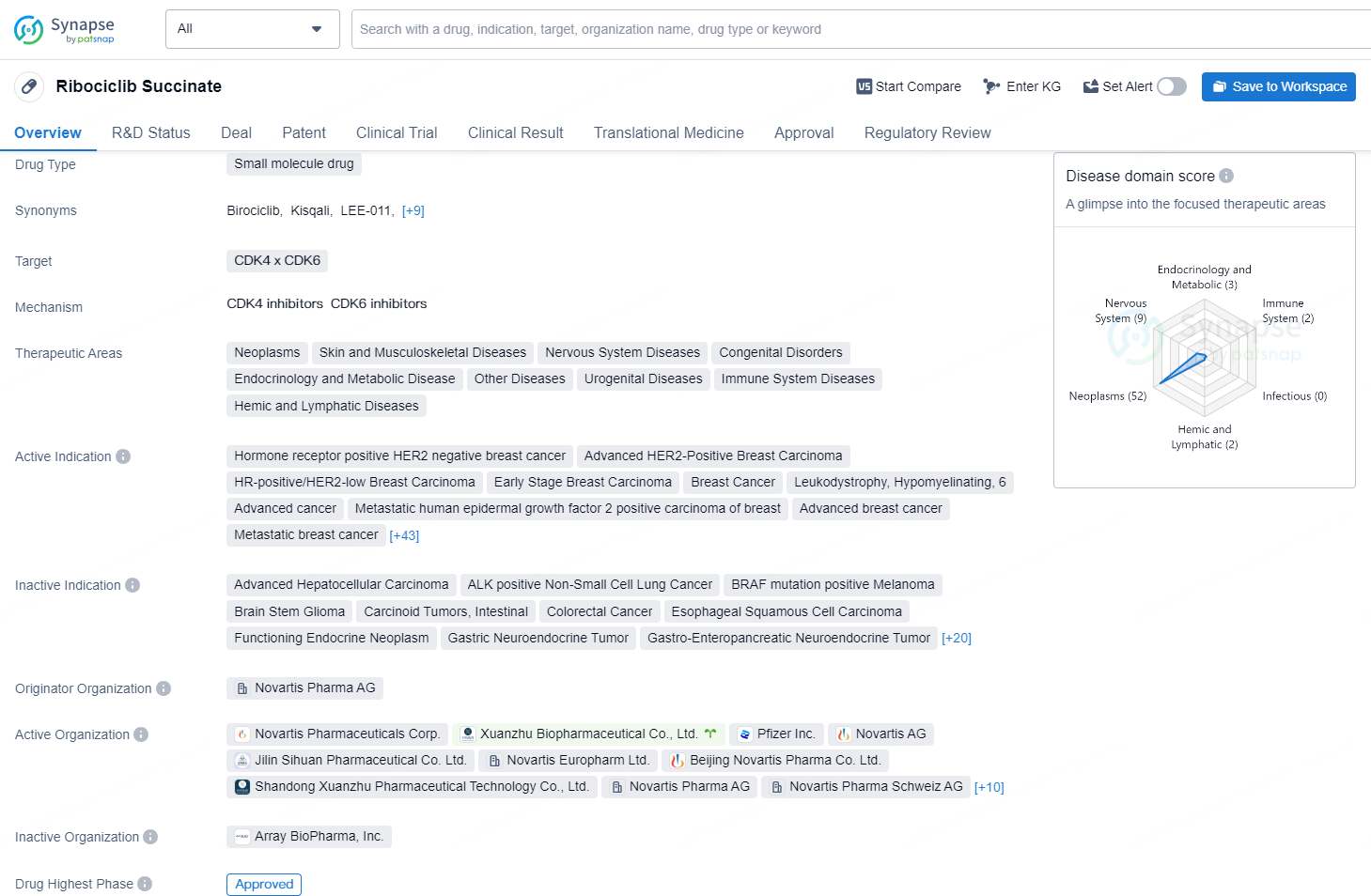
Ribociclib Succinate is a small molecule drug that targets CDK4 and CDK6 and has been approved for various therapeutic areas including neoplasms, skin and musculoskeletal diseases, nervous system diseases, congenital disorders, endocrinology and metabolic disease, other diseases, urogenital diseases, immune system diseases, and hemic and lymphatic diseases.