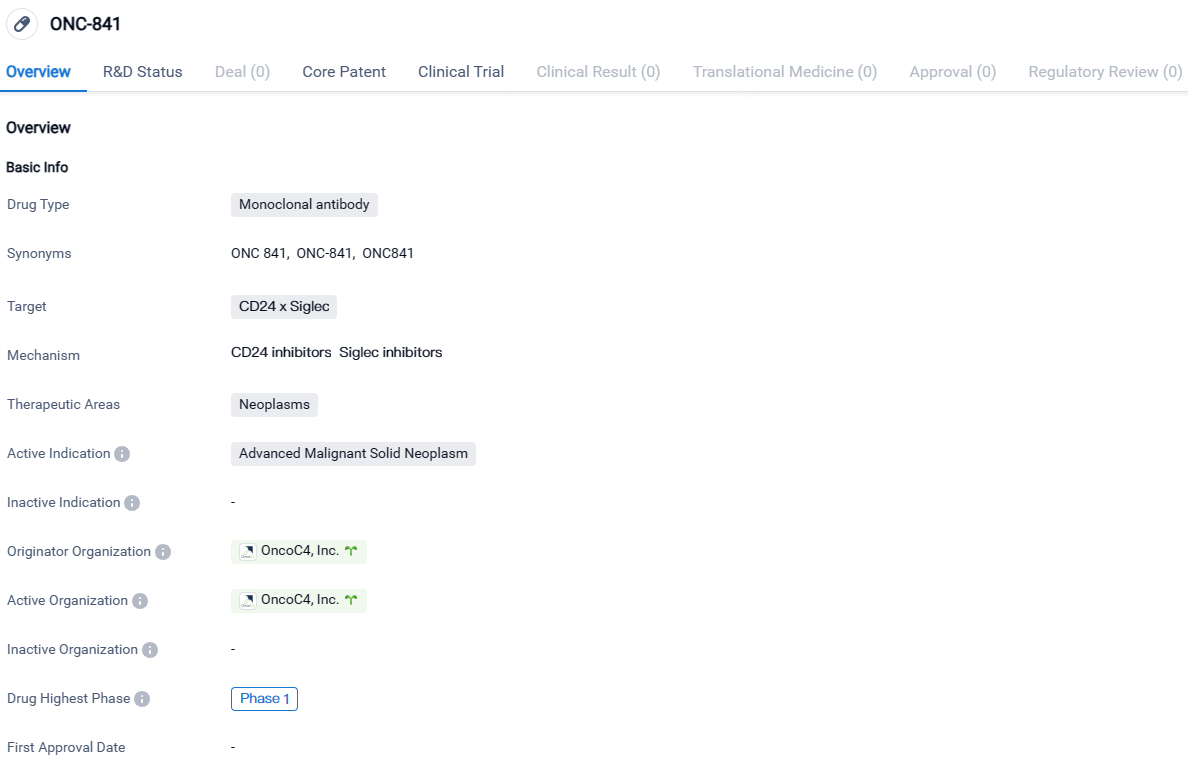
FDA Approves OncoC4's IND for SIGLEC 10 Blocker ONC-841 Against Solid Tumors
OncoC4, Inc., an advanced-stage biopharmaceutical firm focused on creating innovative cancer treatments, has revealed that its new drug application for ONC-841, a pioneering SIGLEC 10 blocking antibody intended for solid tumor therapy, has been approved by the United States Food and Drug Administration.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"SIGLEC 10 serves as a regulatory barrier in immune responses, curbing the activity of both innate and adaptive immune cells. This mechanism can suppress anti-cancer immunity within the tumor microenvironment, providing a haven where cancer cells can proliferate," explained Yang Liu, PhD, the Co-Founder, CEO, and Chief Scientific Officer of OncoC4. “Our drug, ONC-841, is engineered to neutralize this checkpoint and revitalize the immune response against tumors present in the TME. Initiating clinical trials for ONC-841 in advanced solid tumors is a promising step forward."
SIGLEC 10 uniquely features a genuine immunoreceptor tyrosine-based inhibitory motif, setting it apart from other Siglecs like SIGLEC 15. ONC-841 is developed as a humanized monoclonal antibody targeting SIGLEC 10, representing the first of its kind to engage in clinical trials to our current knowledge.
Research by OncoC4 revealed that SIGLEC 10 plays a role in downregulating antibody-dependent cell-mediated cytotoxicity along with its other established roles. The development of ONC-841 was guided by its potential to amplify antibody-dependent CD16a signaling, which crucially affects the activities of natural killer cells and macrophages, pivotal in targeting tumors.
In the preclinical stage for the IND (Investigational New Drug) application, data showed ONC-841 enhances the engulfment of cancer cells by phagocytes and bolsters the efficacy of the immune cells infiltrating tumors. Moreover, studies using syngeneic and xenograft tumor models validated that ONC-841 treatment leads to a robust immune rejection of tumorous cells. Preliminary results from Phase 1 regarding safety, pharmacokinetics, and the therapeutic efficacy of ONC-841 as a single-agent therapy are anticipated in the latter half of 2025.
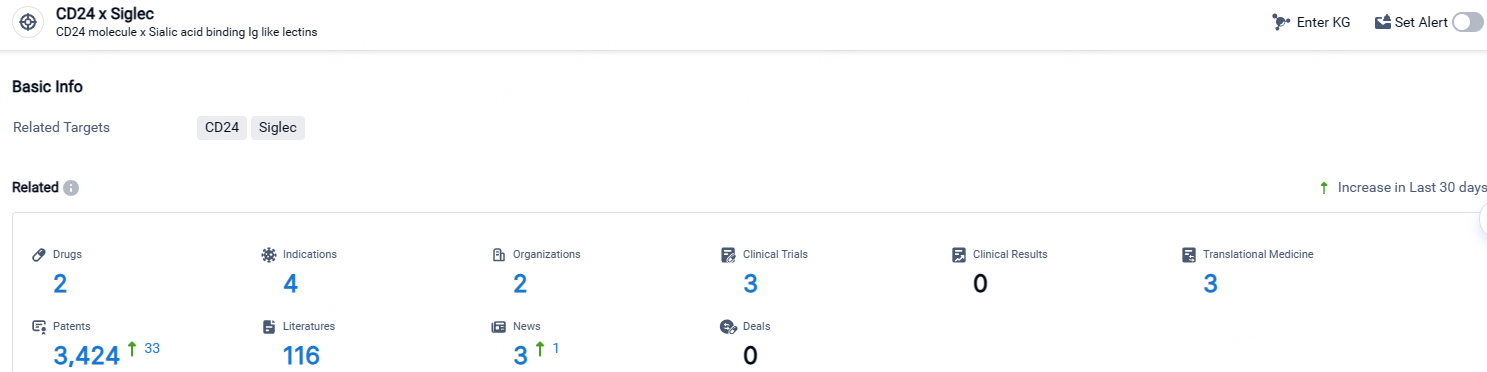
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of April 26, 2024, there are 2 investigational drugs for the CD24 and Siglec target, including 4 indications, 2 R&D institutions involved, with related clinical trials reaching 3, and as many as 3424 patents.
ONC-841 targets the CD24 x Siglec complex and is intended for the treatment of advanced malignant solid neoplasms. Currently in Phase 1 of clinical development, ONC-841 shows promise as a potential therapeutic option for patients with these types of cancers.