Promising Early Results for ALTO-101, Alto Neuroscience's New Schizophrenia Drug
Alto Neuroscience, Inc. revealed encouraging findings from its Phase 1 trial conducted on healthy participants, which focused on ALTO-101, an innovative PDE4 inhibitor being developed to tackle cognitive deficits linked to schizophrenia.
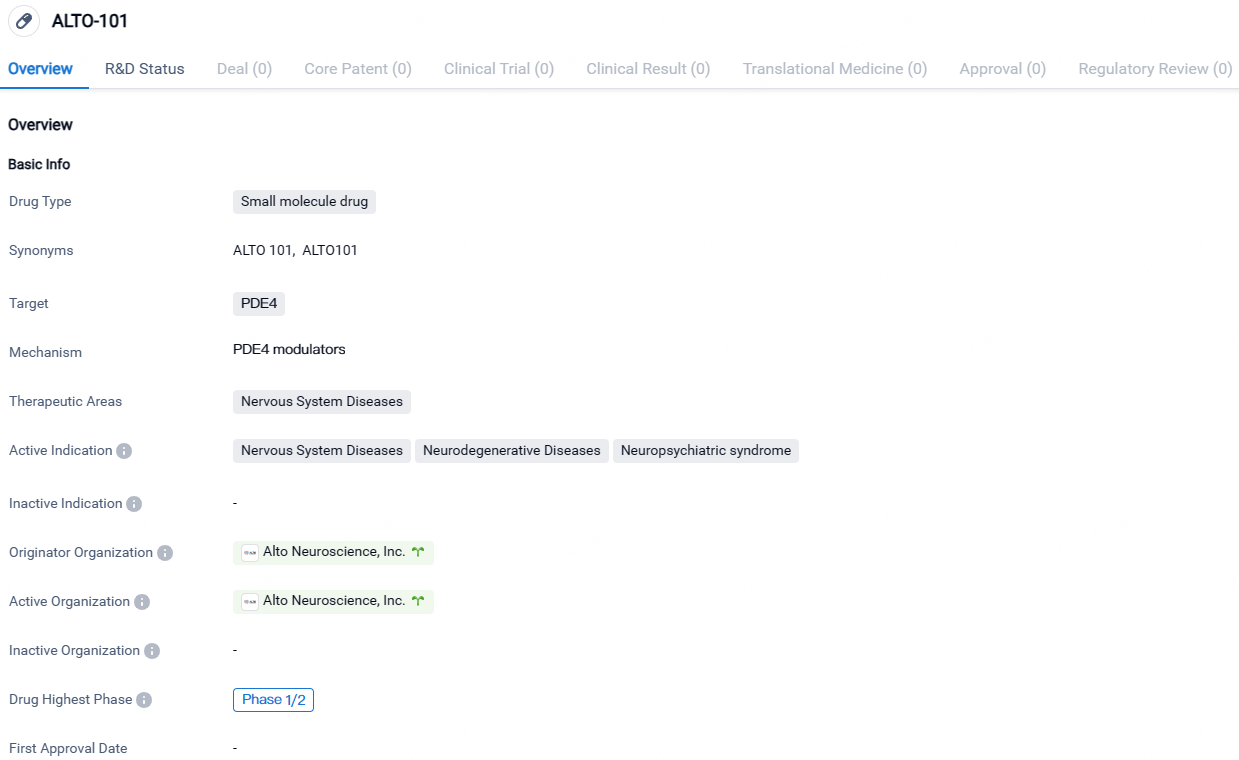
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The development of the TDS formulation is proceeding through a collaborative effort with MEDRx. The drug levels attained through the transdermal approach surpassed those from oral administration, significantly enhancing systemic exposure while minimizing common side effects associated with the drug class. The company is gearing up to launch a proof-of-concept trial to assess ALTO-101 in patients with CIAS during the early months of 2024, expecting main results in the latter half of 2025.
“These encouraging findings strongly suggest that ALTO-101 has potential as a key therapy across various applications. While oral PDE4 inhibitors are recognized for their cognitive benefits, they often come with severe and limiting side effects. Yet, despite these issues of dosage and tolerance, PDE4 inhibitors are frequently prescribed for non-CNS conditions, usually in less-than-ideal dosing schedules,” mentioned Amit Etkin, M.D., Ph.D., the founder and CEO of Alto Neuroscience.
Amit Etkin also noted, “Our studies have shown that the transdermal delivery of ALTO-101 provides a major improvement in maintaining consistent drug levels with a daily formulation that patients tolerate well. The observed decrease in frequent adverse reactions typically associated with this class makes ALTO-101 a notably distinct candidate compared to currently available or under-development PDE4 inhibitors. We are hopeful as we push this treatment forward for CIAS patients, who currently have limited alternatives.”
From the outcomes of this Phase 1 trial, ALTO-101 displays a promising profile with enhanced drug exposure expected to fall within the essential therapeutic range and reduced instances of side effects. Previous reports by the company on the pharmacodynamic effects of orally administered ALTO-101 help define the effective therapeutic range necessary for the sought-after cognitive and clinical benefits.
According to the partnership agreement with MEDRx, Alto is set to make a milestone payment of $1.5 million to MEDRx after achieving the desired pharmacokinetic properties for ALTO-101. This payment will be made through a mix of cash and common stock of Alto Neuroscience.
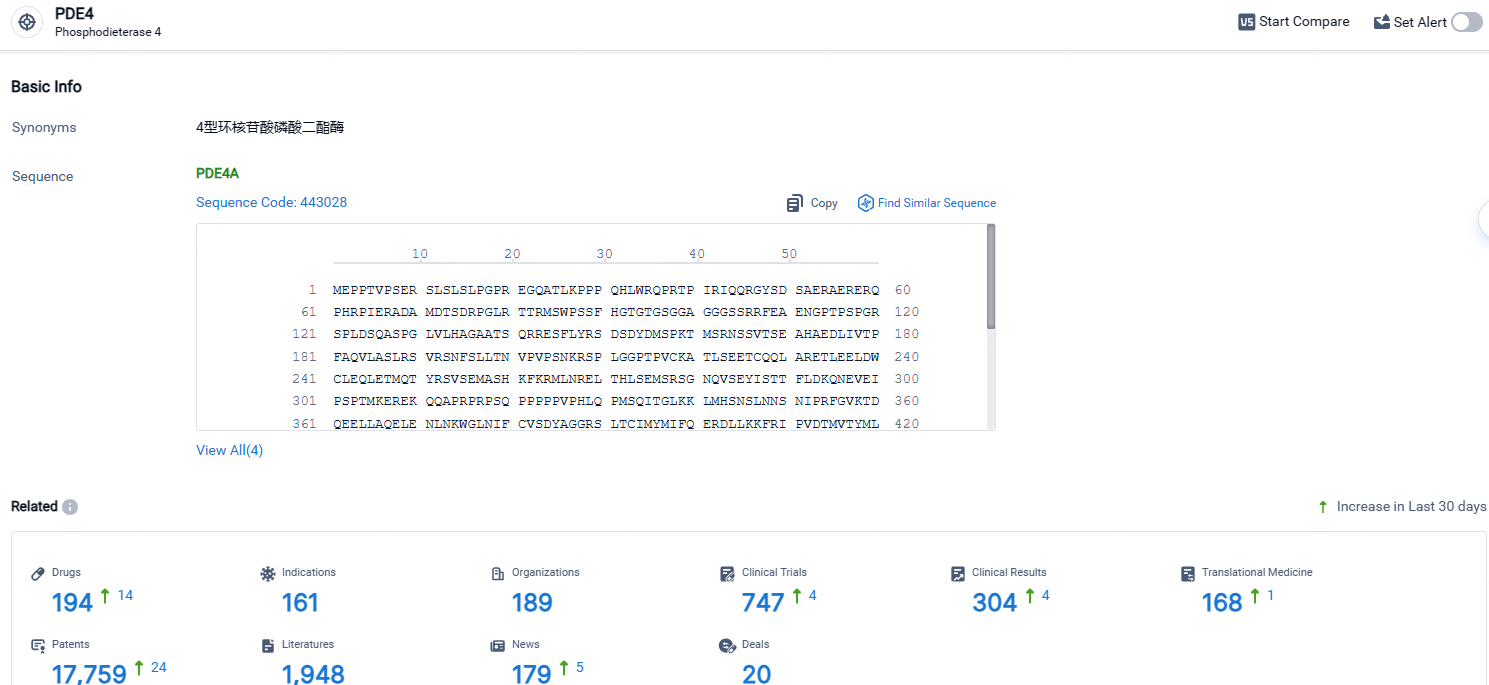
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of April 25, 2024, there are 194 investigational drugs for the PDE4 targets, including 161 indications, 189 R&D institutions involved, with related clinical trials reaching 747, and as many as 17759 patents.
ALTO-101 targets PDE4 and is indicated for the treatment of Nervous System Diseases, including Neurodegenerative Diseases and Neuropsychiatric syndromes. The drug is currently in Phase 1/2 of clinical development, and its originator organization is Alto Neuroscience, Inc.