FDA Approves Opdivo® with Chemotherapy for Pre- and Post-Surgery Treatment in Resectable NSCLC
Bristol Myers Squibb (NYSE: BMY) disclosed that their Opdivo®(nivolumab) has received approval from the U.S. Food and Drug Administration (FDA) for use in adult patients with resectable non-small cell lung cancer (NSCLC) whose tumors are 4 cm or larger or have positive lymph nodes. This treatment excludes cases with known epidermal growth factor receptor (EGFR) mutations or anaplastic lymphoma kinase (ALK) rearrangements. The approval covers its use as a neoadjuvant therapy combined with platinum-doublet chemotherapy, followed by Opdivo alone as an adjuvant treatment post-surgery. This overall approach is termed perioperative therapy, involving administration before and after surgical procedures.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The authorization is based on findings from the CheckMate-77T study, which marks the company’s second successful Phase 3 randomized trial featuring an immunotherapy-centered combination for treating resectable NSCLC. Currently, Opdivo stands as the sole PD-1 inhibitor to exhibit statistically significant and clinically relevant advantages in this disease compared to chemotherapy, both in a neoadjuvant-only regimen and as an element of a perioperative regimen.
"Considering the disease recurrence rates in patients with resectable NSCLC, there’s a clear demand for treatments that can be used pre- and post-surgery, potentially addressing micrometastasis, reducing cancer recurrence risk, and enhancing the likelihood of successful surgical intervention," stated Dr. Tina Cascone, associate professor of Thoracic/Head and Neck Medical Oncology at The University of Texas MD Anderson Cancer Center.
"This endorsement represents progress for patients with resectable conditions, as the perioperative nivolumab with neoadjuvant chemotherapy regimen can lead to better event-free survival (EFS) compared to neoadjuvant chemotherapy alone and offers the potential for achieving a pathologic response (pCR) in 25% of patients."
The CheckMate-77T trial scrutinized the perioperative regimen of neoadjuvant Opdivo with platinum-doublet chemotherapy succeeded by surgery and adjuvant Opdivo monotherapy (n=229), contrasting with neoadjuvant platinum-doublet chemotherapy and placebo followed by surgery and adjuvant placebo (n=232) in adult patients with resectable NSCLC. In this trial, the Opdivo group showed improved EFS, a key endpoint, compared to the chemotherapy and placebo group. A notable pCR rate was also observed, serving as one of the predefined secondary endpoints.
The likelihood of disease recurrence, progression, or death was cut down by 42% (EFS Hazard Ratio [HR] 0.58; 95% Confidence Interval [CI]: 0.43 to 0.78; P=0.00025) in patients assigned to the Opdivo group, in comparison to the chemotherapy and placebo group, with a median follow-up period of 25.4 months. Additionally, 18-month EFS was seen in 70% of patients within the Opdivo group, compared to 50% within the chemotherapy and placebo group. Moreover, 25% of individuals in the Opdivo group reached pCR, whereas 4.7% in the control group attained pCR in the intent-to-treat cohort (estimated treatment difference of 20.5%; 95% CI, 14.3 to 26.6).
Opdivo is linked with these Warnings & Precautions: severe and potentially fatal immune-mediated adverse events, such as pneumonitis, colitis, hepatitis and hepatotoxicity, endocrinopathies, dermatologic reactions, nephritis and renal dysfunction; reactions related to infusion; complications from allogeneic hematopoietic stem cell transplantation (HSCT); embryo-fetal toxicity. The use of PD-1 or PD-L1 blocking antibodies together with a thalidomide analogue and dexamethasone for multiple myeloma patients is advised against outside of rigorously controlled clinical studies. Please refer to Important Safety Information below.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
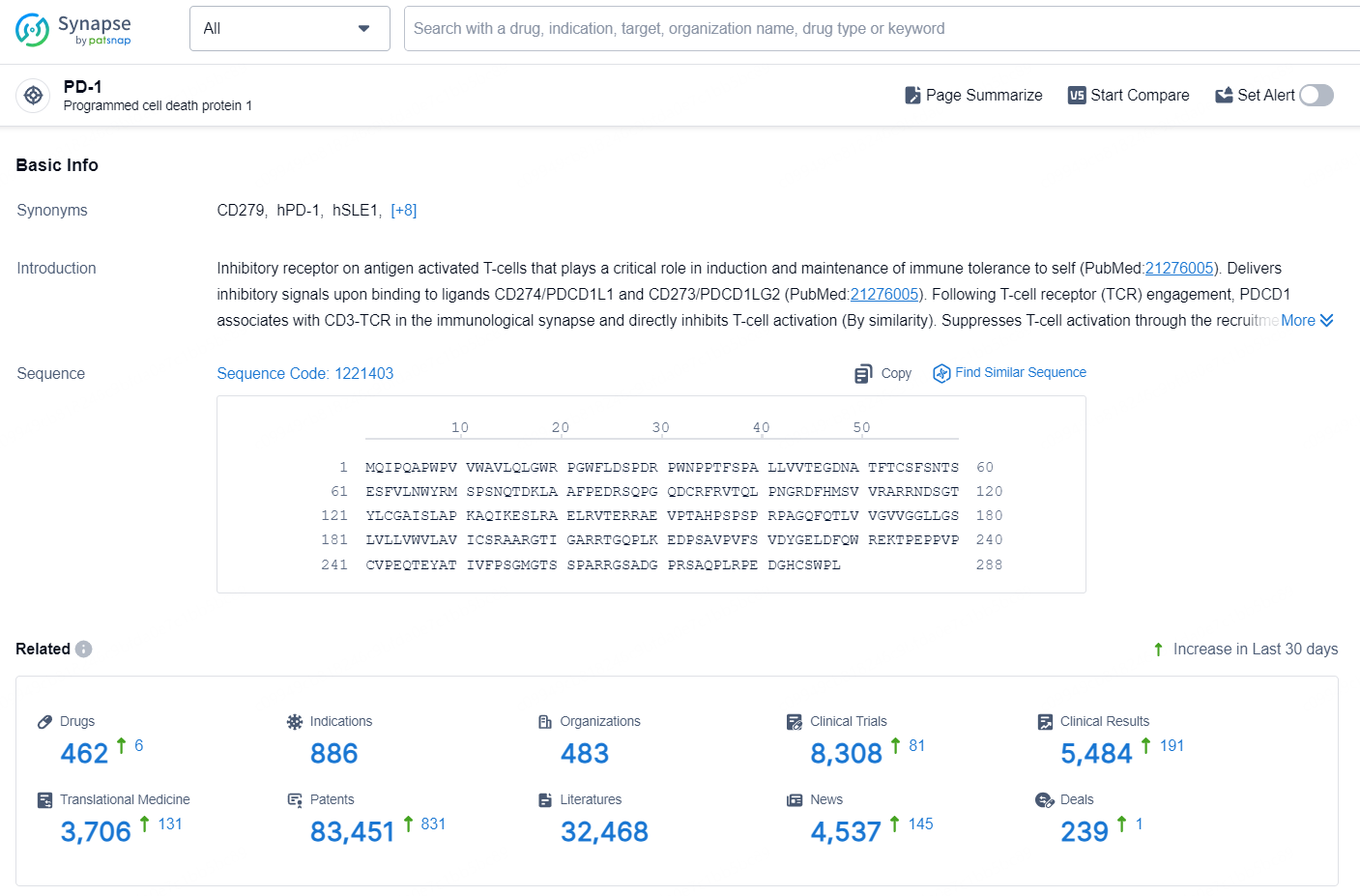
According to the data provided by the Synapse Database, As of October 9, 2024, there are 462 investigational drugs for the PD-1 target, including 886 indications, 483 R&D institutions involved, with related clinical trials reaching 8308, and as many as 83451 patents.
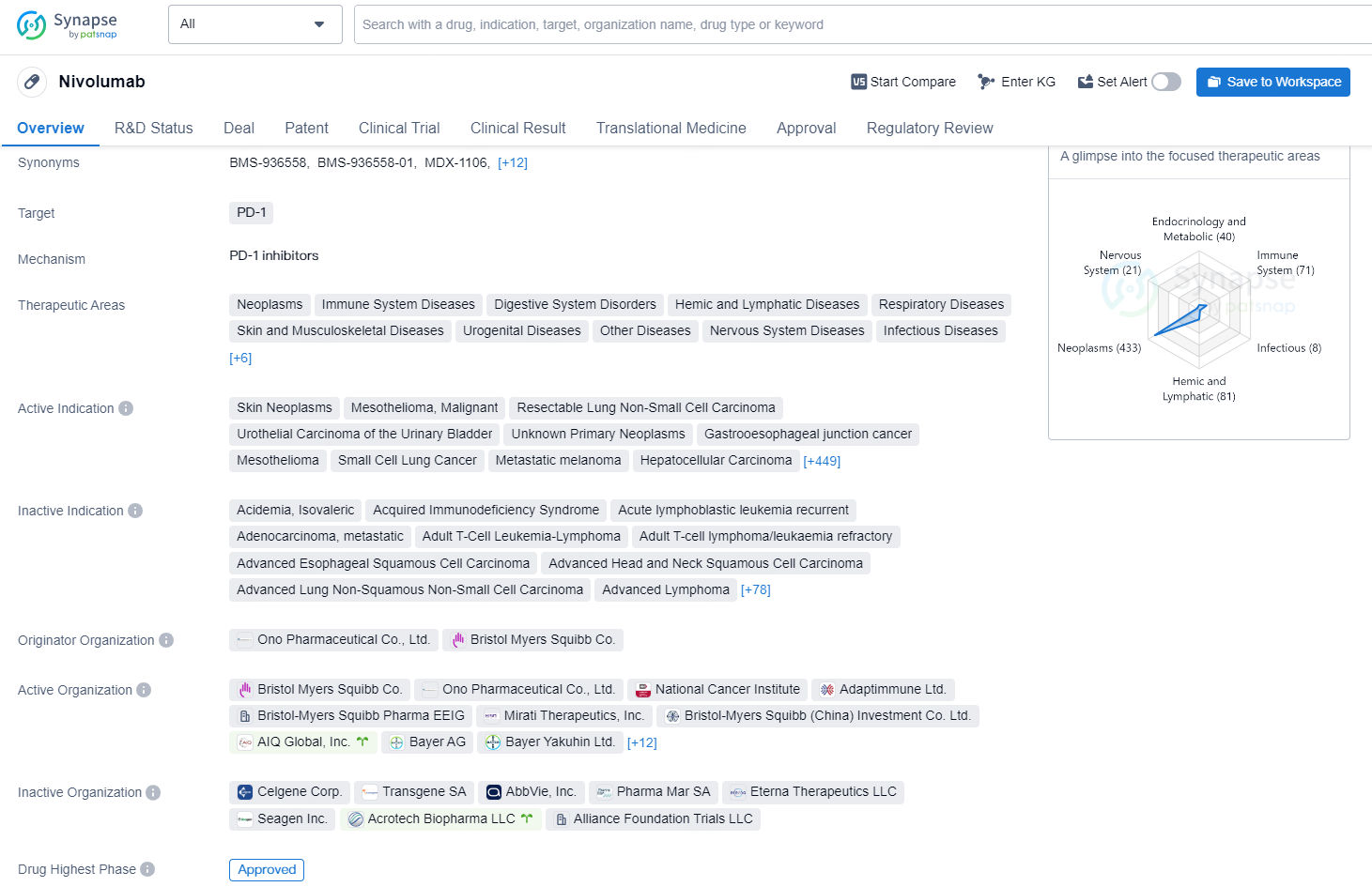
Nivolumab is a monoclonal antibody drug that targets PD-1, with therapeutic areas that include a wide range of neoplasms, immune system diseases, digestive system disorders, and various other diseases. The drug is approved for treating skin neoplasms, mesothelioma, resectable lung non-small cell carcinoma, urothelial carcinoma of the urinary bladder, and various other types of cancers as well as different diseases.