Lasting Effects of SC TALVEY® and DARZALEX® in Relapsed or Refractory Multiple Myeloma
Janssen-Cilag International NV, under the umbrella of Johnson & Johnson, reported revised findings from the experimental Phase 1b TRIMM-2 trial. This study assesses the efficacy of a combination treatment involving TALVEY®▼ (talquetamab), DARZALEX® (daratumumab) in its subcutaneous (SC) formulation, and pomalidomide in patients with relapsed or refractory multiple myeloma. The results indicated an overall response rate (ORR) of 82 percent, thereby underscoring the potential of this therapeutic combination. These findings were presented during an oral session at the 2024 International Myeloma Society (IMS) Annual Meeting, held in Rio de Janeiro, Brazil, from September 25 to 28 (Abstract #OA-01).
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The findings of the Phase 1b TRIMM-2 trial assessing talquetamab, the pioneering bispecific T-cell engager targeting GPRC5D, in combination with subcutaneous daratumumab, the first subcutaneous anti-CD38 monoclonal antibody, and pomalidomide, included participants who had undergone at least three previous lines of therapy, incorporating a proteasome inhibitor (PI) and an immunomodulatory drug (IMiD), or were double refractory to both PI and IMiD and had not received anti-CD38 therapy in the last 90 days.
“In an era where CD38-targeting therapies are becoming increasingly prevalent in initial treatment settings, it is crucial to continue exploring novel approaches for those managing multiple myeloma in later treatment stages, regardless of prior treatment history,” stated Edmond Chan, MBChB, M.D. (Res), EMEA Therapeutic Area Lead Haematology, Innovative Medicine, Johnson & Johnson. “Our commitment remains steadfast in leveraging the promising potential of this talquetamab regimen as part of our ongoing mission to revolutionize patient outcomes and ultimately eradicate cancer.”
At the time of the data cutoff, 77 patients had been administered talquetamab at doses of either 0.4 mg/kg weekly (QW) or 0.8 mg/kg biweekly (Q2W), accompanied by step-up doses, combined with subcutaneous daratumumab and pomalidomide. The QW cohort (n=18) exhibited a 100 percent overall response rate (ORR), with 56 percent attaining a complete response (CR) or better.1 In the Q2W group (n=59), the ORR stood at 76 percent, with 56 percent reaching CR or better.1 The median duration of response (DOR) in the Q2W group was 26.4 months, and the median progression-free survival (PFS) was 20.3 months.1 Data also indicated that 51.6 percent of patients refractory to anti-CD38 (n=64) achieved CR or better, whereas 70.8 percent of those with prior chimeric antigen receptor T-cell (CAR-T) therapy (n=24) achieved CR or better. In addition, patients previously treated with bispecific antibodies (n=29) had an 82.8 percent ORR.
“The deep and sustained responses revealed by the latest TRIMM-2 results further underscore the potential of talquetamab in combination with subcutaneous daratumumab, now a standard of care for multiple myeloma, and pomalidomide,” commented Nizar Bahlis, M.D., Associate Professor at the Arnie Charbonneau Cancer Institute of the University of Calgary and presenting author. “With significant overall response rates observed across patient cohorts, this combination shows promise for robust disease management and extended survival in patients who have been treated with numerous prior therapies, including those exposed to previous bispecific antibodies.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
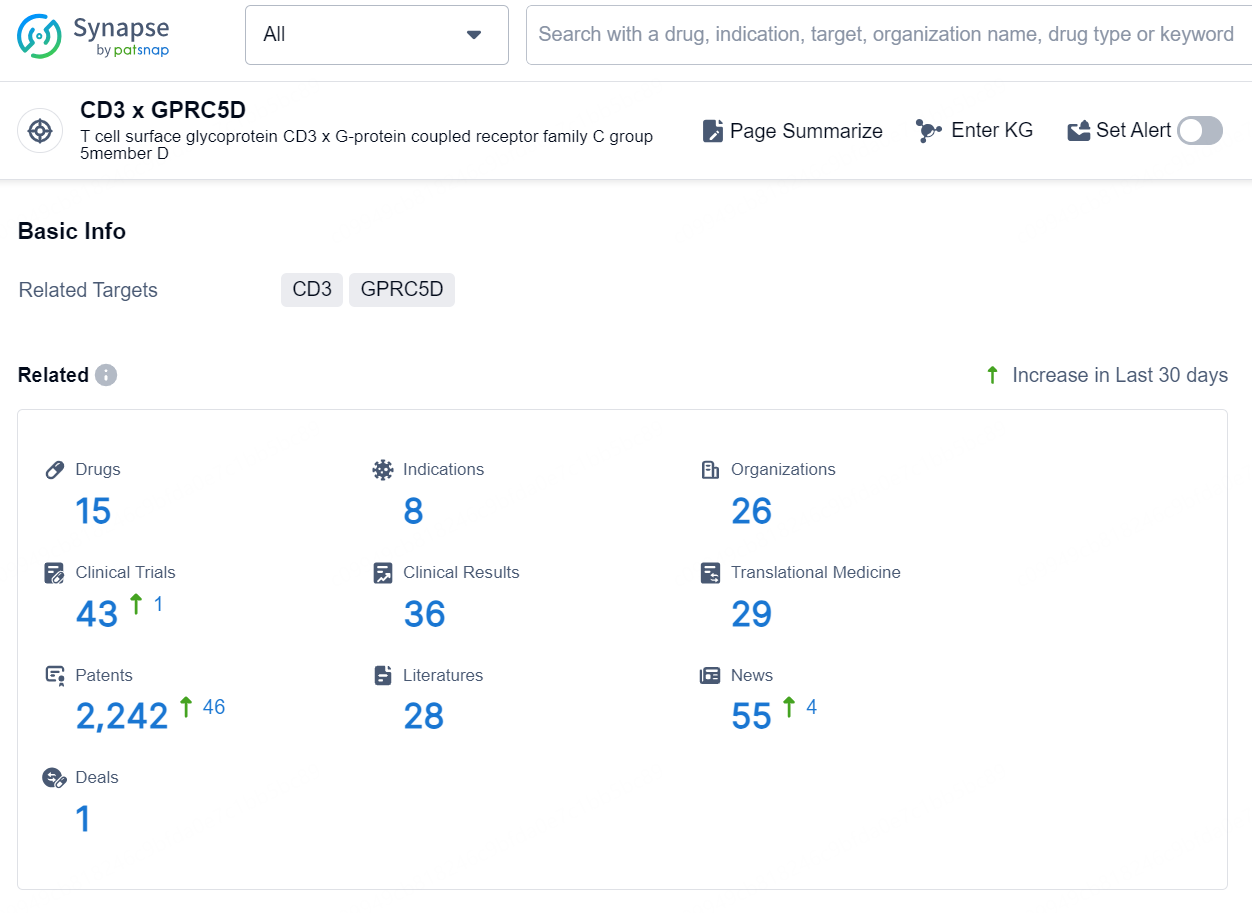
According to the data provided by the Synapse Database, As of September 30, 2024, there are 15 investigational drusg for the CD3 x GPRC5D targets, including 8 indications, 26 R&D institutions involved, with related clinical trials reaching 43, and as many as 2242 patents.
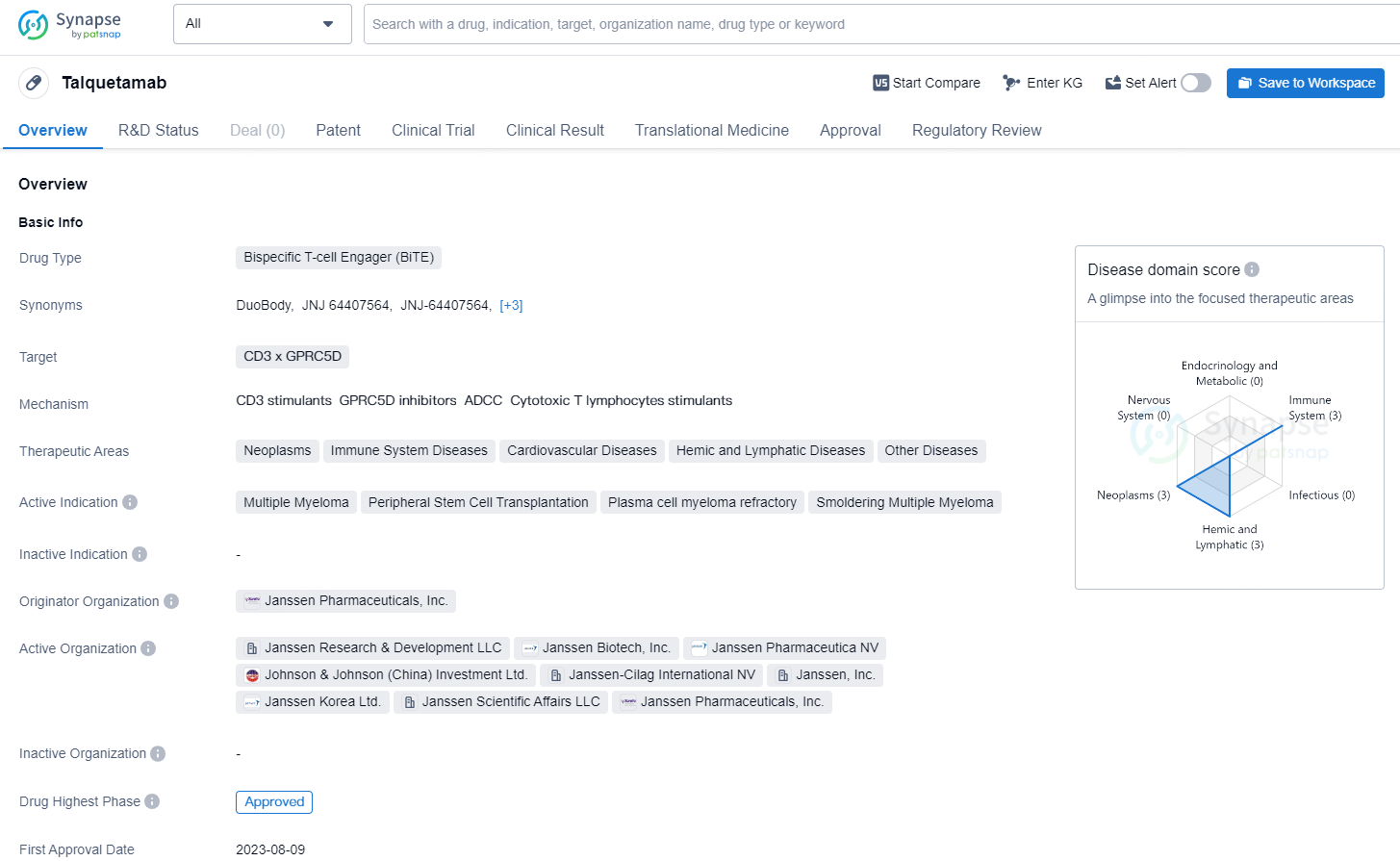
Talquetamab is a bispecific T-cell Engager (BiTE) drug developed by Janssen Pharmaceuticals, Inc. The drug targets CD3 x GPRC5D and is indicated for the treatment of various conditions, including neoplasms, immune system diseases, cardiovascular diseases, hemic and lymphatic diseases, and other diseases. Its active indications include multiple myeloma, peripheral stem cell transplantation, plasma cell myeloma refractory, and smoldering multiple myeloma.