FDA approves priority review of Odronextamab BLA for relapsed/refractory follicular and diffuse large B-cell lymphoma
Regeneron Pharmaceuticals, Inc. has reported that the U.S. FDA has accepted the Priority Review for its BLA for odronextamab. This investigational therapy is aimed at treating adults who have suffered relapses or are resistant to treatment for either follicular lymphoma or R/R diffuse large B-cell lymphoma and have had insufficient progress after a minimum of two systemic therapies.
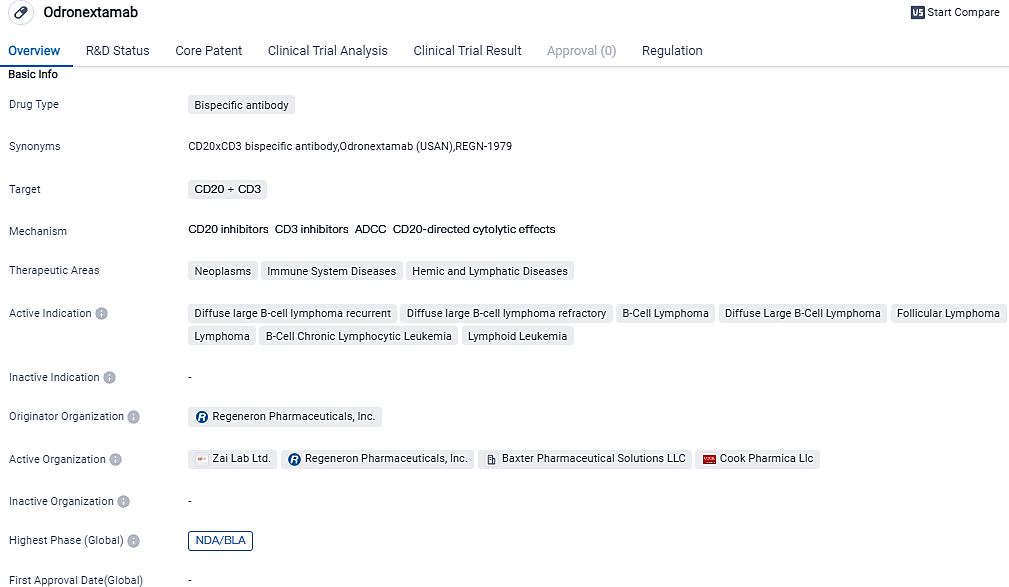
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The target action date for the FDA decision is March 31, 2024. Odronextamab, an experimental CD20xCD3 bispecific antibody under investigation, is engineered to connect CD20 on cancer cells with CD3-expressing T cells, thus enhancing local T-cell activation and bolstering the destruction of cancer cells.
Supporting data from a Phase 1 and crucial Phase 2 trial backed the BLA. The most recent results, discussing the research on odronextamab for both FL and DLBCL, were shared at the 64th American Society of Hematology Annual Meeting.
In past, the FDA has conferred Fast Track Designation and Orphan Drug Designation to odronextamab for FL and DLBCL. In the month of August, the European Medicines Agency accepted a Marketing Authorization Application for the review of odronextamab to treat adult patients with R/R FL or R/R DLBCL who have shown progress after at least two earlier systemic therapies.
FL and DLBCL are two of the most frequently occurring subtypes of B-cell non-Hodgkin lymphoma. FL is a slowly progressing subtype and although initial treatments are successful for numerous patients, it is estimated that nearly 20% may suffer a relapse within two years, experiencing reduced remissions with each subsequent therapy.
DLBCL is a more aggressive subtype, with nearly half of the high-risk patients showing progress after the first-line treatment. As these blood cancers develop further, the treatment becomes increasingly difficult, particularly in the third-line setting and beyond, thereby leaving patients with limited treatment options.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of October 9, 2023, there are 31 investigational drugs for the CD20+CD3 target, including 35 indications, 32 R&D institutions involved, with related clinical trials reaching 159,and as many as 13337 patents.
Odronextamab appears to be a promising drug candidate in the field of biomedicine. Its bispecific antibody nature, targeting both CD20 and CD3, suggests a potential for enhanced efficacy in treating the indicated diseases. Odronextamab is currently under clinical development, and its safety and efficacy have not been fully evaluated by any regulatory authority.