FDA Approves Samsung Bioepis' EPYSQLI® as Soliris Biosimilar
Samsung Bioepis Co., Ltd. revealed today that the U.S. Food and Drug Administration has granted approval for the Biologics License Application for EPYSQLI® (eculizumab-aagh), recognizing it as a biosimilar to Soliris (eculizumab).
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
 EPYSQLI has received regulatory approval for the management of patients with paroxysmal nocturnal hemoglobinuria to mitigate hemolysis, and for those with atypical hemolytic uremic syndrome to restrain complement-mediated thrombotic microangiopathy. EPYSQLI is not authorized for the treatment of individuals with Shiga toxin E. coli-associated hemolytic uremic syndrome.
EPYSQLI has received regulatory approval for the management of patients with paroxysmal nocturnal hemoglobinuria to mitigate hemolysis, and for those with atypical hemolytic uremic syndrome to restrain complement-mediated thrombotic microangiopathy. EPYSQLI is not authorized for the treatment of individuals with Shiga toxin E. coli-associated hemolytic uremic syndrome.
The FDA’s endorsement of EPYSQLI is grounded on a comprehensive body of evidence, encompassing analytical, non-clinical, and clinical data that validate its close similarity to Soliris, with no substantial clinical differences between EPYSQLI and Soliris regarding safety, purity, and potency.
A randomized Phase I, double-blind, three-arm, parallel group, single-dose trial in healthy subjects established pharmacokinetic equivalence and comparable pharmacodynamic, safety, tolerability, and immunogenicity profiles between EPYSQLI and Soliris. Furthermore, a randomized Phase 3, double-blind, multicenter, cross-over trial in PNH patients confirmed clinical parity in efficacy, safety, PK, and immunogenicity between the two treatments.
"Our commitment has always been to enhance patients' lives by delivering high-quality, safe, and effective biological medicines. Our efforts now extend to rare disease domains where patients encounter limited access to vital therapies," stated Christopher Hansung Ko, President and Chief Executive Officer at Samsung Bioepis.
Eculizumab, a monoclonal antibody and anti-C5 complement inhibitor, is a renowned standard therapy for PNH and aHUS, rare conditions affecting approximately 50,000 and 5,000 individuals in the United States, respectively. It is observed that around 70% of PNH patients on eculizumab do not follow the prescribed dosage regimen, and two-thirds discontinue the treatment within 1.5 years, possibly due to factors such as high treatment expenses.
Beyond the US, EPYSQLI has also been sanctioned by the European Commission and Korea’s Ministry of Food and Drug Safety as a biosimilar to Soliris for the treatment of PNH and aHUS. In regions where EPYSQLI holds approval and availability, it may not be prescribed or dispensed for other indications for which Soliris is sanctioned.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
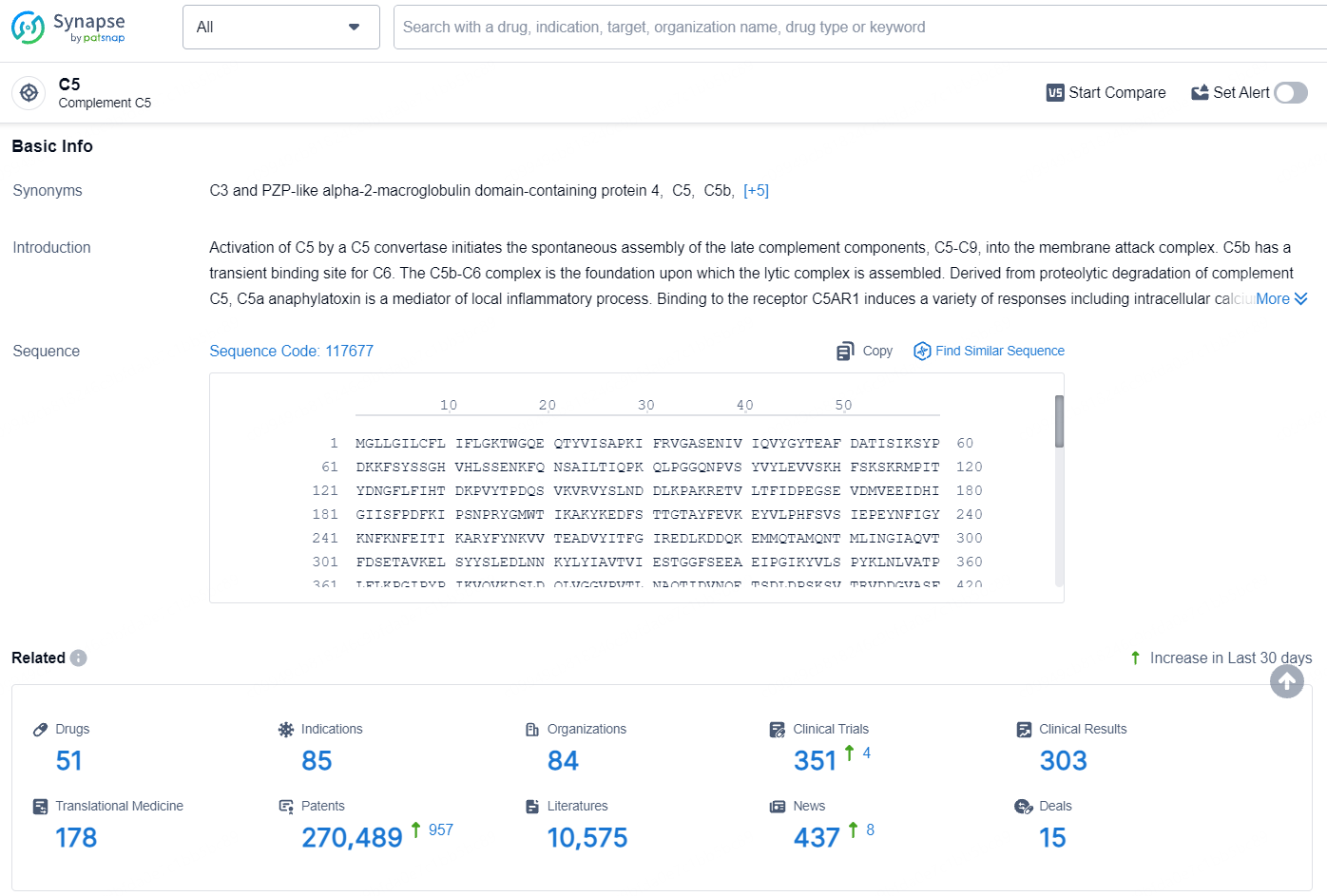
According to the data provided by the Synapse Database, As of July 25, 2024, there are 51 investigational drugs for the C5 target, including 85 indications, 84 R&D institutions involved, with related clinical trials reaching 351, and as many as 270489 patents.
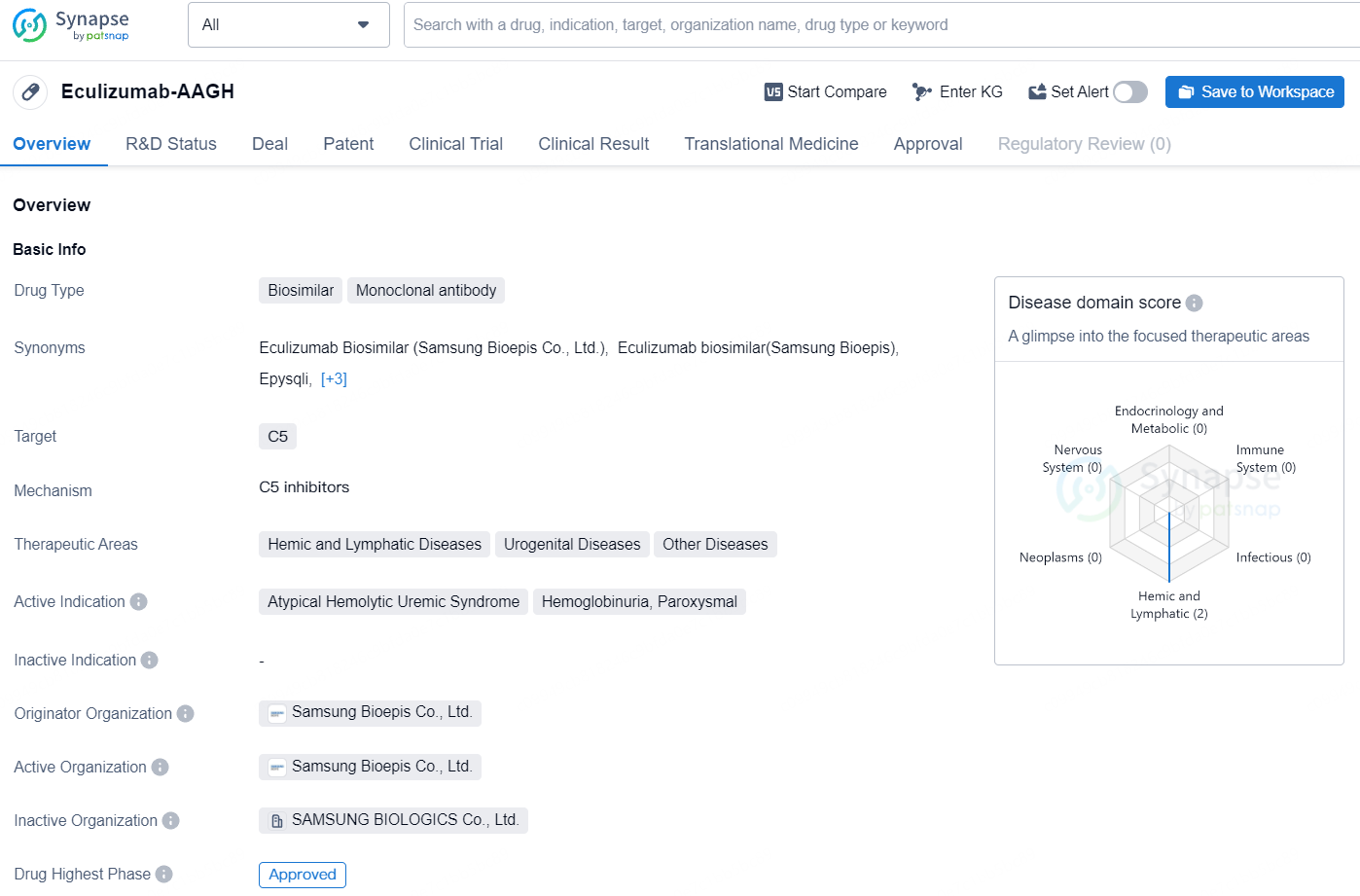
Eculizumab-AAGH is a biosimilar monoclonal antibody drug that targets the C5 protein and is indicated for various hemic, lymphatic, and urogenital diseases. It has been approved in the European Union, Iceland, Liechtenstein, and Norway, with the first approval date recorded in May 2023. However, its development and availability in China have been discontinued, suggesting limitations to its use in that market.