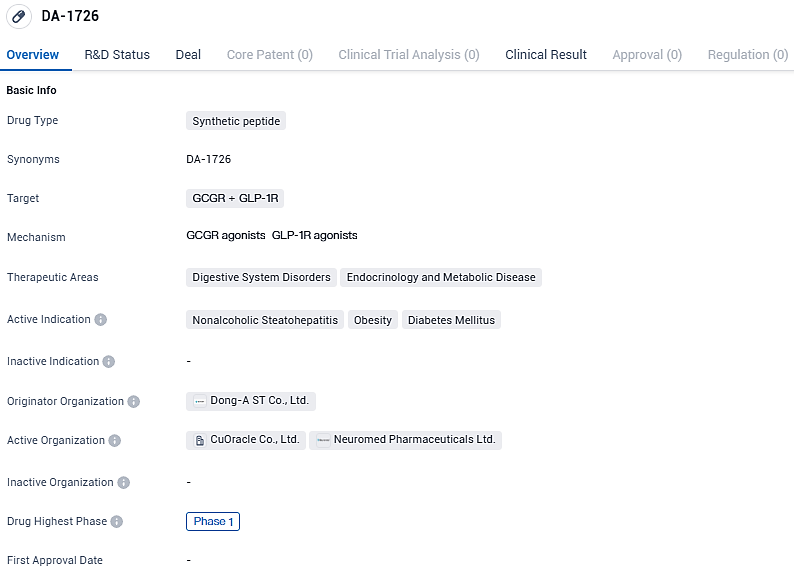
NeuroBo Pharma files IND with FDA for Phase 1 trial of anti-obesity compound DA-1726
NeuroBo Pharmaceuticals, Inc., a company specializing in the advancement of biotechnological treatments for cardiometabolic disorders at the clinical development phase, disclosed its recent submission of an Investigational New Drug (IND) application to the U.S. Food and Drug Administration (FDA). This submission paves the way for an inaugural Phase 1 clinical study on DA-1726, an innovative, dual-acting oxyntomodulin derivative that effectively stimulates both the glucagon-like peptide-1 receptor (GLP1R) and the glucagon receptor (GCGR), targeting the therapeutic area of obesity.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Submission of the Investigational New Drug (IND) application for DA-1726 marks a pivotal moment in the advancement of this GLP-1 and glucagon dual receptor agonist, propelling its progress towards clinical trials as a promising candidate to tackle the substantial obesity sector. This was articulated by NeuroBo's President and CEO, Hyung Heon Kim.
Kim added, "We are of the opinion that the symmetrical stimulation of GLP-1 and glucagon receptors by DA-1726 could result in improved regulation of blood sugar levels and may offer a more favorable safety profile compared to existing GLP-1 receptor agonists. We are eager to commence the clinical trials for DA-1726, with an anticipation of administering the initial dose in the latter half of 2024 and projecting an analysis of data in the latter half of 2025."
The Phase 1 study is structured as a randomized, placebo-controlled, double-blind, parallel group design with sequential enrollment, aiming to evaluate the safety profile, dose tolerability, pharmacokinetic characteristics, and pharmacodynamic effects of both single and multiple escalations in dose levels of DA-1726 administered to overweight but otherwise healthy individuals.
The main focus for the trial will be the monitoring of DA-1726's safety and tolerance by documenting the incidence of adverse and serious adverse events, as well as any adverse reactions that emerge during treatment and those that necessitate discontinuation of the therapy. Secondary objectives entail the pharmacokinetics of DA-1726, gauged by serum levels over a duration and analysis of metabolites at higher doses. Additional exploratory objectives will investigate the impact of DA-1726 on various metabolic and heart-related markers, fasting blood fats, weight, waist measurement, and body mass index, among other factors.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
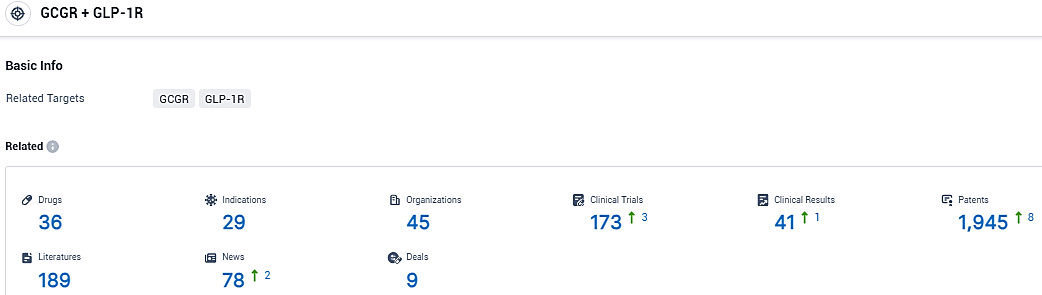
According to the data provided by the Synapse Database, As of January 4, 2024, there are 36 investigational drugs for the GCGR and GLP-1R target, including 29 indications, 45 R&D institutions involved, with related clinical trials reaching 41, and as many as 1945 patents.
DA-1726 is a novel oxyntomodulin analogue functioning as a GLP1R/GCGR dual agonist for the treatment of obesity and NASH that is to be administered once weekly subcutaneously. DA-1726 as a dual agonist of GLP-1 receptors and glucagon receptors, leading to weight loss through reduced appetite and increased energy expenditure. DA-1726 has a well understood mechanism and, in preclinical mice models, resulted in improved weight loss compared to semaglutide and cotadutide .