FDA Approves Zepbound® for Obstructive Sleep Apnea in Obese Adults
Eli Lilly and Company (NYSE: LLY) has received approval from the U.S. Food and Drug Administration (FDA) for Zepbound® (tirzepatide), marking it as the inaugural and sole prescription treatment for adults suffering from moderate-to-severe obstructive sleep apnea (OSA) in conjunction with obesity. Zepbound may aid adults facing these conditions in enhancing their sleep issues. It is recommended to be used alongside a calorie-restricted diet and an increase in physical activity levels.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"Often, obstructive sleep apnea (OSA) is dismissed as 'just snoring,' but it encompasses much more than that," stated Julie Flygare, J.D., president and CEO of Project Sleep. "Recognizing OSA symptoms and being aware of available treatments, including innovative options like Zepbound, is crucial. We hope this ignites more substantial discussions between patients and their healthcare providers, ultimately leading to improved health outcomes."
OSA is a disorder related to sleep that involves full or partial obstruction of the upper airway during sleep, potentially resulting in breathing interruptions (apnea) or shallow breaths (hypopnea), which can cause a drop in oxygen levels and/or awakenings during sleep. While snoring is a significant symptom of OSA, fatigue, excessive daytime drowsiness, and disrupted sleep patterns also play critical roles, making this condition often underestimated.
"Currently, a significant number of OSA cases remain undiagnosed and untreated, placing millions at risk for severe health issues," remarked Patrik Jonsson, executive vice president, and president of Lilly Cardiometabolic Health and Lilly USA. "Zepbound is the first medication that significantly enhances outcomes for moderate-to-severe OSA and contributes to sustained weight loss in obese adults. Nearly half of the participants in clinical trials experienced such improvements that they no longer exhibited symptoms of OSA, representing a vital advancement in alleviating the impact of this disorder and its related health concerns."
This approval was granted based on the findings from the SURMOUNT-OSA phase 3 clinical trials, which assessed Zepbound (10 mg or 15 mg) for treating moderate-to-severe OSA in adults with obesity, with and without positive airway pressure (PAP) therapy over one year. Zepbound proved to be approximately five times more effective than the placebo at minimizing breathing interruptions in adults not using PAP therapy, leading to a reduction of 25 interruptions per hour with Zepbound, compared to five with the placebo. For adults using PAP therapy, Zepbound resulted in 29 fewer interruptions per hour versus six with the placebo. After a year, 42% of participants using Zepbound and 50% of those combining Zepbound with PAP therapy achieved remission or non-symptomatic mild OSA, compared to 16% and 14% in the placebo group, respectively.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
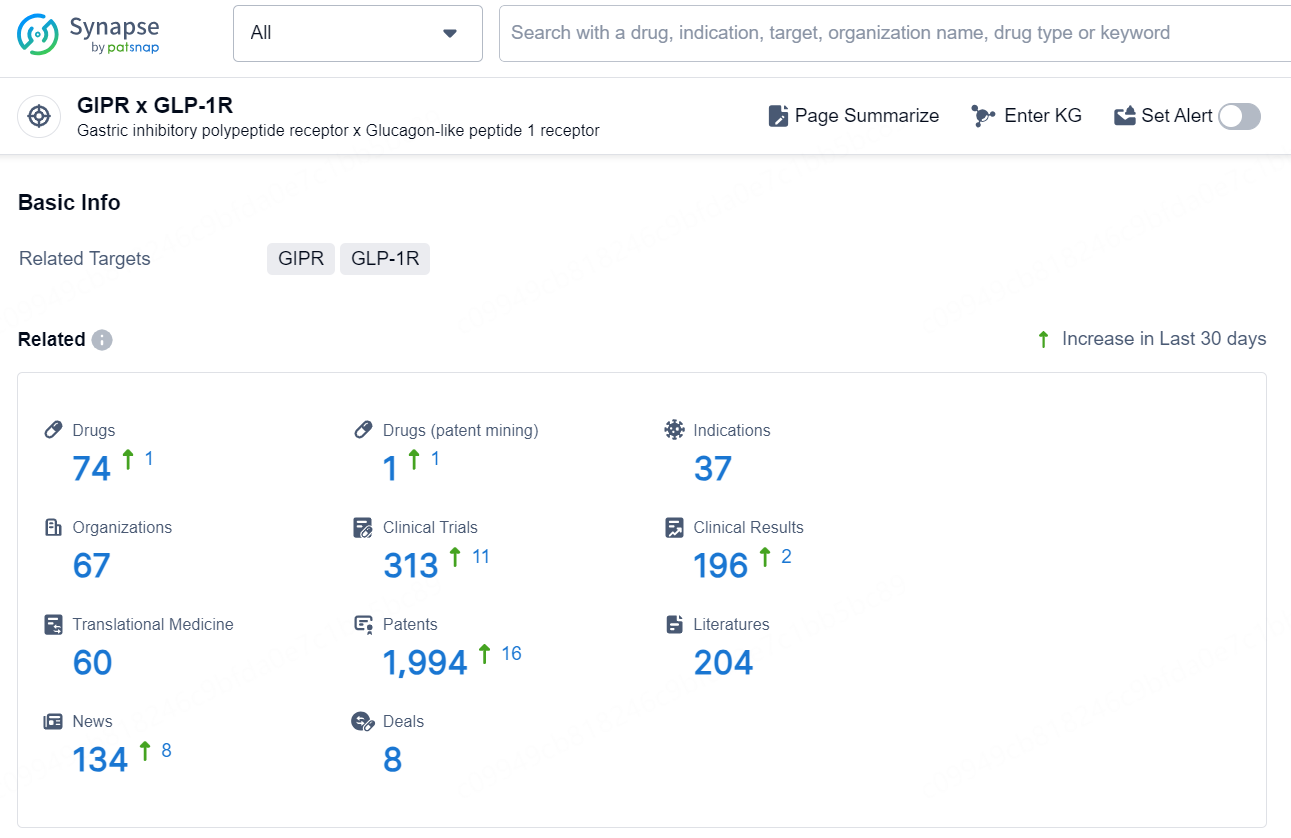
According to the data provided by the Synapse Database, As of December 30, 2024, there are 74 investigational drugs for the GIPR x GLP-1R target, including 37 indications, 67 R&D institutions involved, with related clinical trials reaching 313, and as many as 1994 patents.
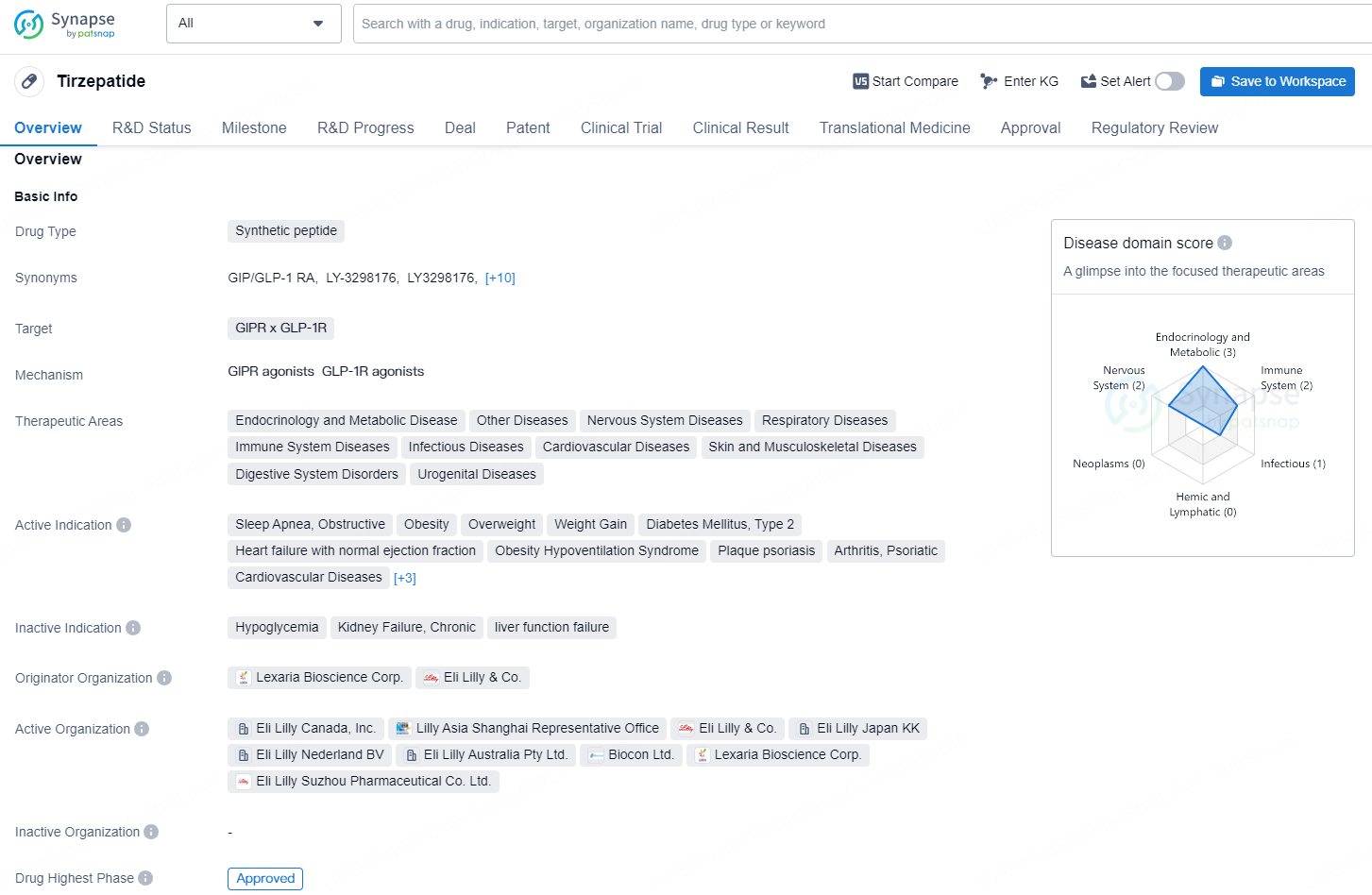
Tirzepatide is a novel medication developed for the treatment of type 2 diabetes and potentially for weight management. It is a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist. This dual action makes Tirzepatide unique compared to other diabetes treatments.