FDA Clears Full-Life Tech's 225Ac-FL-020 for Treating Metastatic Castration-Resistant Prostate Cancer
Full-Life Technologies, an all-encompassing international radiotherapeutics firm, has recently revealed that the U.S. Food and Drug Administration has approved its Investigational New Drug Application. This approval pertains to the clinical trials of 225Ac-FL-020, a radiopharmaceutical aimed at PSMA for the treatment of metastatic castration-resistant prostate cancer. The company intends to commence clinical trials both in the United States and internationally in 2024.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
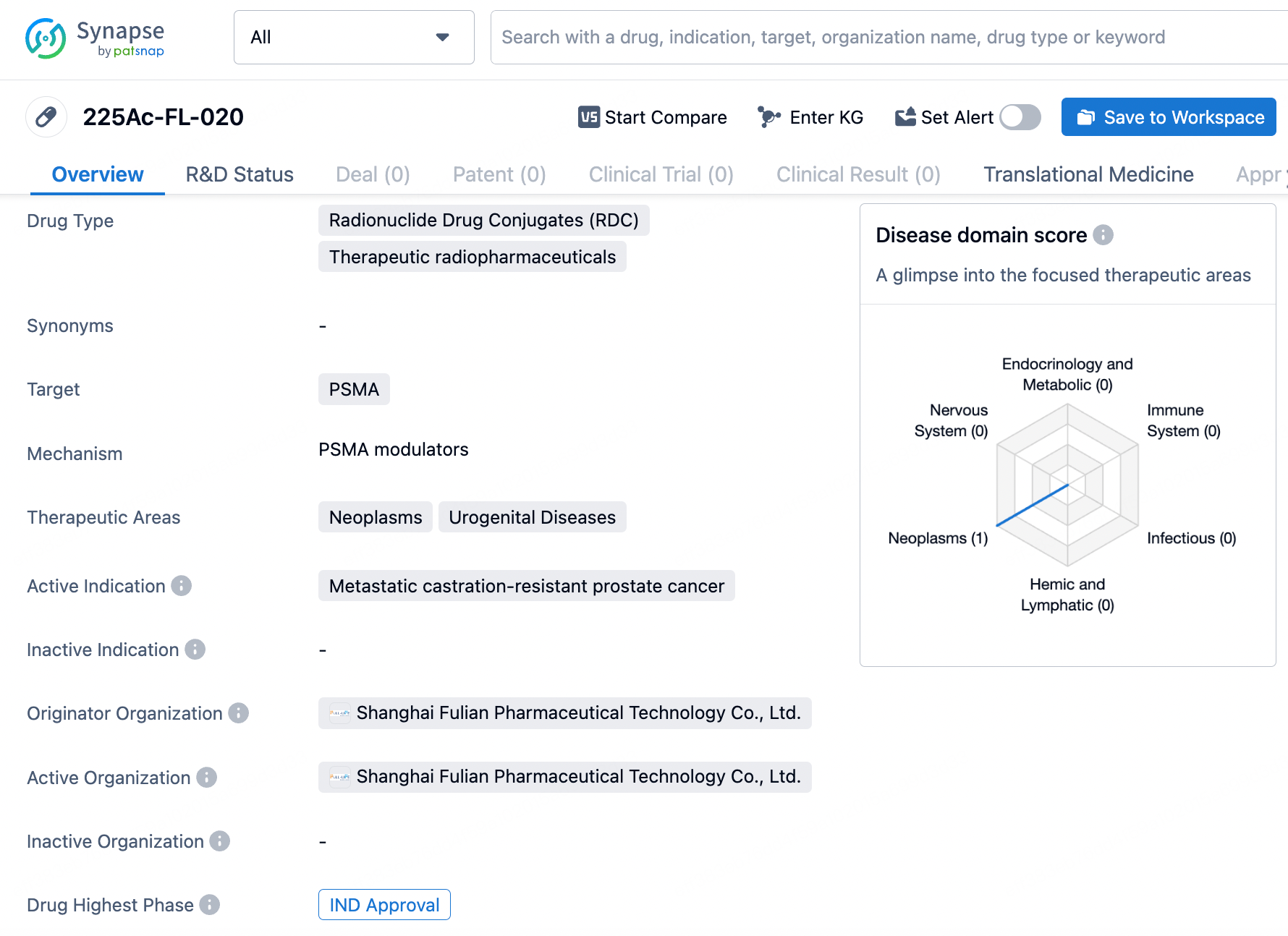
225Ac-FL-020 utilizes targeted alpha-radiotherapy to selectively destroy cancerous cells, thereby minimizing harm to normal tissues. In preclinical studies, radiolabeled FL-020 showed exceptional in vivo biodistribution, characterized by significant and prolonged accumulation in tumors and rapid clearance from the body.
In LNCaP xenograft mice, 225Ac-FL-020 demonstrated strong anti-tumor effects with an acceptable safety profile. The forthcoming Phase I clinical trial will assess 225Ac-FL-020’s safety, tolerability, and anti-cancer efficacy, establishing a foundation for subsequent clinical studies and potentially positioning 225Ac-FL-020 as a key treatment for patients with mCRPC.
“The clearance of the IND application signifies a crucial regulatory achievement in our developmental trajectory for 225Ac-FL-020,” remarked Steffen Heeger, M.D., M.Sc., Chief Medical Officer of Full-Life. “This milestone emphasizes our dedication to the therapeutic promise of radiopharmaceuticals and the diligence and interdisciplinary cooperation of our team. We eagerly anticipate the initiation of the Phase I clinical program, which will provide our first human data on the safety and anti-tumor potential of 225Ac-FL-020.”
225Ac-FL-020 represents a promising, next-generation PSMA-targeting radionuclide drug conjugate set to commence global Phase I clinical trials in 2024. The FL-020 targeting vector was discovered through Full-Life’s exclusive UniRDC™ platform, significantly enhancing drug uptake in tumors while ensuring rapid systemic elimination. Preclinical models have shown that 225Ac-FL-020 possesses strong anti-tumor properties and a favorable safety profile.
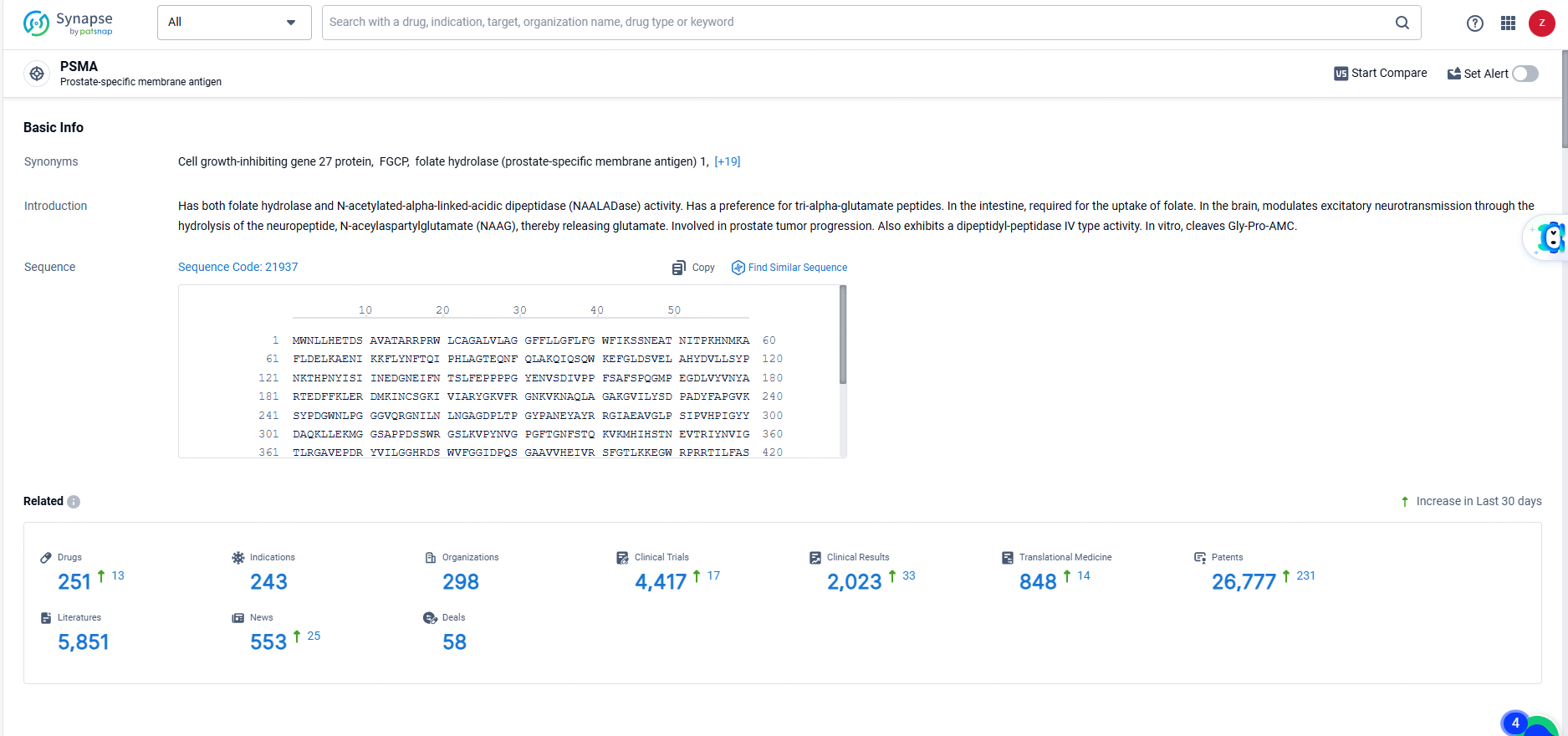
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of June 3, 2024, there are 251 investigational drugs for the PSMA targets, including 243 indications, 298 R&D institutions involved, with related clinical trials reaching 4417, and as many as 26777 patents.
225Ac-FL-020 holds promise as a targeted therapeutic option for a challenging and life-threatening condition, and its advancement through the development process will be of interest to stakeholders in the pharmaceutical and healthcare industries.