FDA fast-tracked Amgen's Tarlatamab for advanced small cell lung cancer treatment
Amgen has disclosed that its submission of the Biologics License Application for tarlatamab to the U.S. Food and Drug Administration has not only been acknowledged but has also received an expedited Priority Review designation from the agency.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
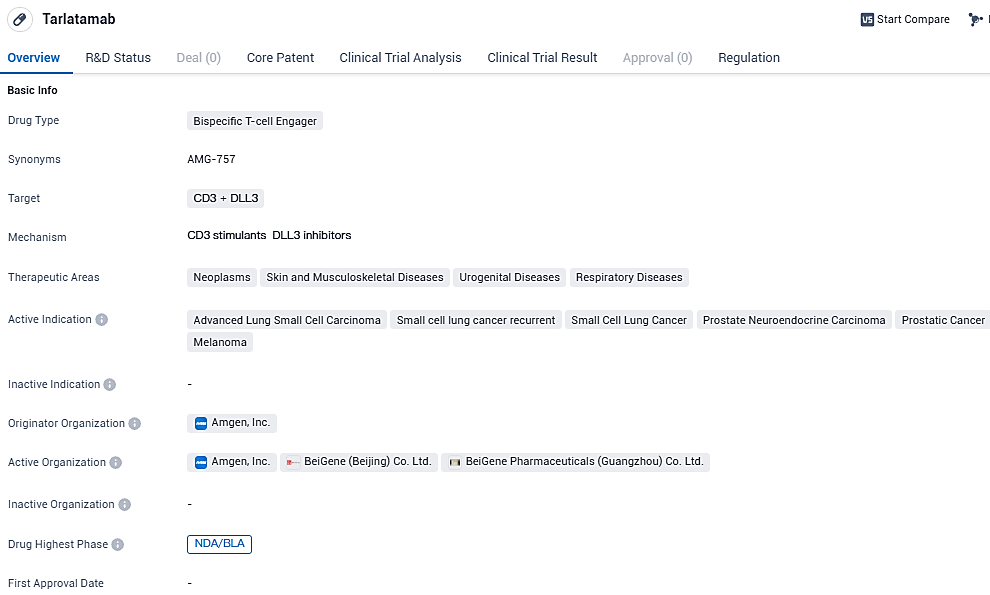
Tarlatamab represents a pioneering therapy as a Bispecific T-cell Engager (BiTE) aimed at the delta-like ligand 3 (DLL3) pathway, currently under exploration as a possible novel treatment for adults with advanced small cell lung cancer (SCLC) who have not responded well to chemotherapy regimens containing platinum compounds.
In recognition of the critical need for fresh therapeutic avenues for those with advanced-stage SCLC who have experienced progression post-platinum-based chemotherapy protocols, the FDA has assigned the Priority Review status to Tarlatamab's application, noted by Amgen's Research and Development executive vice president, Dr. David M. Reese.
Although initial therapies may yield substantial initial benefits, the recurrence of SCLC can be notably aggressive, making enduring survival outcomes elusive. Dr. Reese highlighted that, once relapse sets in, the scarcity of further viable treatment strategies amplifies the dire need for innovative treatments for individuals confronting this late-stage condition.
This Priority Review status, awarded by the FDA, signals potentially considerable advancements in treatment efficacy compared to existing modalities or suggests a potentially new therapeutic pathway for conditions currently lacking satisfactory treatments. With the Priority Review in place, the tarlatamab Prescription Drug User Fee Act (PDUFA) goal date is anticipated to be on or before June 12, 2024.
Previously, in October, tarlatamab received the FDA's Breakthrough Therapy Designation, reinforcing its potential. The ongoing review process encompasses the FDA's Project Orbis initiative and Real Time Oncology Review program, aimed at expediting the availability of oncology medications through collective multi-nation submissions, a testament to the FDA Oncology Center of Excellence's commitment to innovation.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
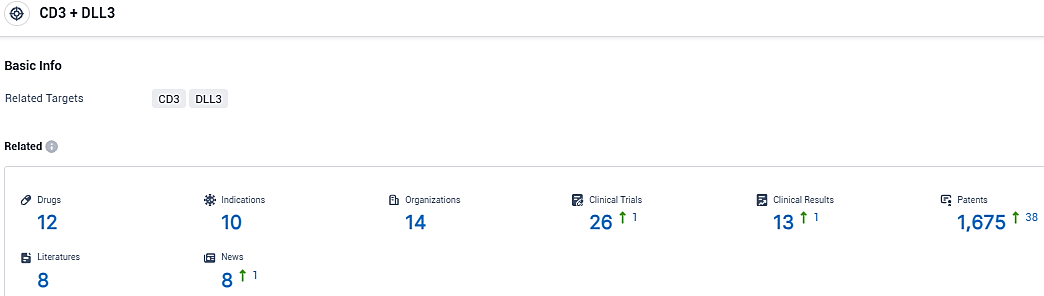
According to the data provided by the Synapse Database, As of December 21, 2023, there are 12 investigational drugs for the CD3 and DLL3 target, including 10 indications, 14 R&D institutions involved, with related clinical trials reaching 26, and as many as 1675 patents.
Tarlatamab is an investigational, targeted therapy engineered by Amgen researchers that brings a patient's own T cells in close proximity to SCLC cells by binding both CD3 on T cells and DLL3 on SCLC cells. This results in the formation of a cytolytic synapse with lysis of the cancer cell.12,13 DLL3 represents an exciting therapeutic target for patients with SCLC, as approximately 85% to 96% of patients have expression of DLL3 on the cell surface of SCLC cells, with minimal expression in normal cells.