FDA Greenlights NMD670 Phase 2b Trial in US Myasthenia Gravis Patients
NMD Pharma A/S has made public the news that the US Food and Drug Administration has granted authorization for their Investigational New Drug submission. This approval permits the company to commence a Phase 2b clinical study of NMD670. The trial will focus on patients who have generalized myasthenia gravis and test positive for either anti-acetylcholine receptor antibodies or anti-muscle-specific tyrosine kinase antibodies.
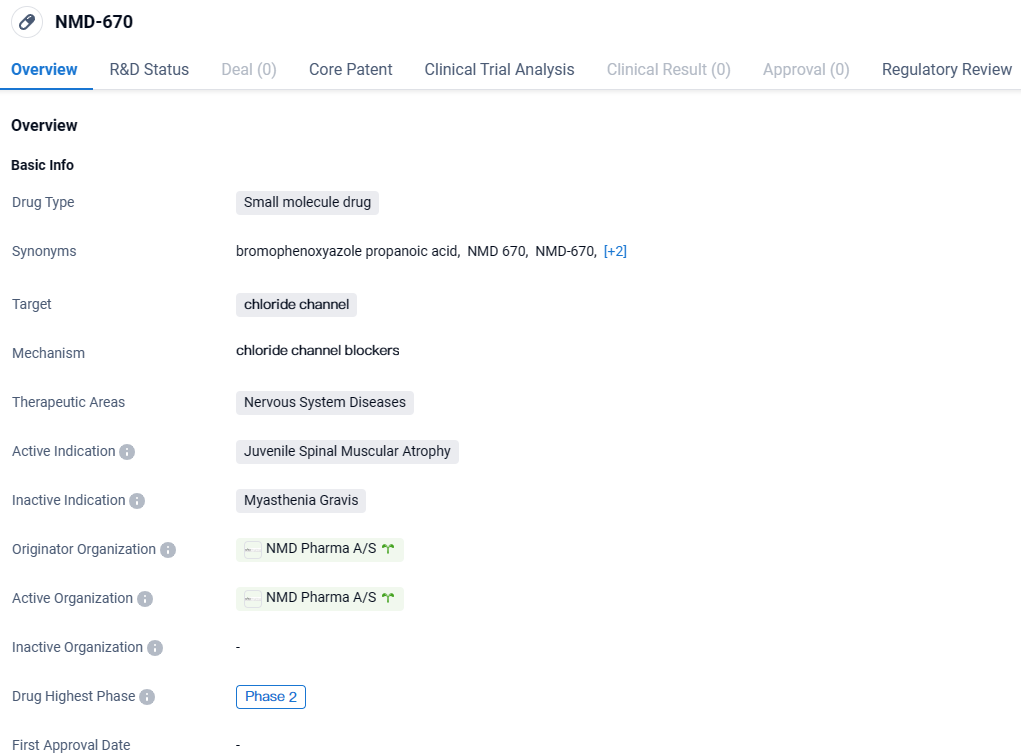
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The upcoming Stage 2b investigation is a methodic assessment aimed at determining the effective dosage of NMD670, an innovative muscle-specific therapy administered orally twice daily. This double-masked, placebo-regulated study is designed to observe the compound's effects in gMG sufferers who continue to exhibit stable or varying signs despite adhering to the established primary treatments. Both European and American medical research facilities will host the trials. The recruitment of participants for this study is slated to begin soon.
NMD670 stands as a pioneering ClC-1 chloride ion channel inhibitor, particularly acting on the skeletal muscle. NMD Pharma recently disclosed encouraging outcomes from its early-stage Phase 1/2a clinical data through an article in Science Translational Medicine. This research underlines the initial clinical verification for the mechanism of CIC-1 inhibition in individuals enduring gMG. It also confirms the safe use and acceptability of NMD670 for these patients.
Thomas Holm Pedersen, the CEO of NMD Pharma, remarked on this significant leap forward, emphasizing the transition of their primary investigational compound, NMD670, into a Stage 2b trial within the US for patients diagnosed with generalized myasthenia gravis and displaying positive indicators for AChR and MuSK antibodies. He expressed enthusiasm about the advancement of a distinct and previously corroborated treatment method concentrated on the muscles. This approach anticipates delivering enhancements in muscular functionality that translate into noticeable benefits for routine activities and overall patient well-being.
As of November 2023, NMD Pharma publicized the acquisition of €75 million via a Series B funding round. These funds are intended to support the completion of three different Stage 2 scientific evaluations involving NMD670, the firm's lead ClC-1 inhibitor, geared towards patients with gMG who test positive for AChR and MuSK antibodies, as well as those affected by spinal muscular atrophy and Charcot-Marie-Tooth disease.
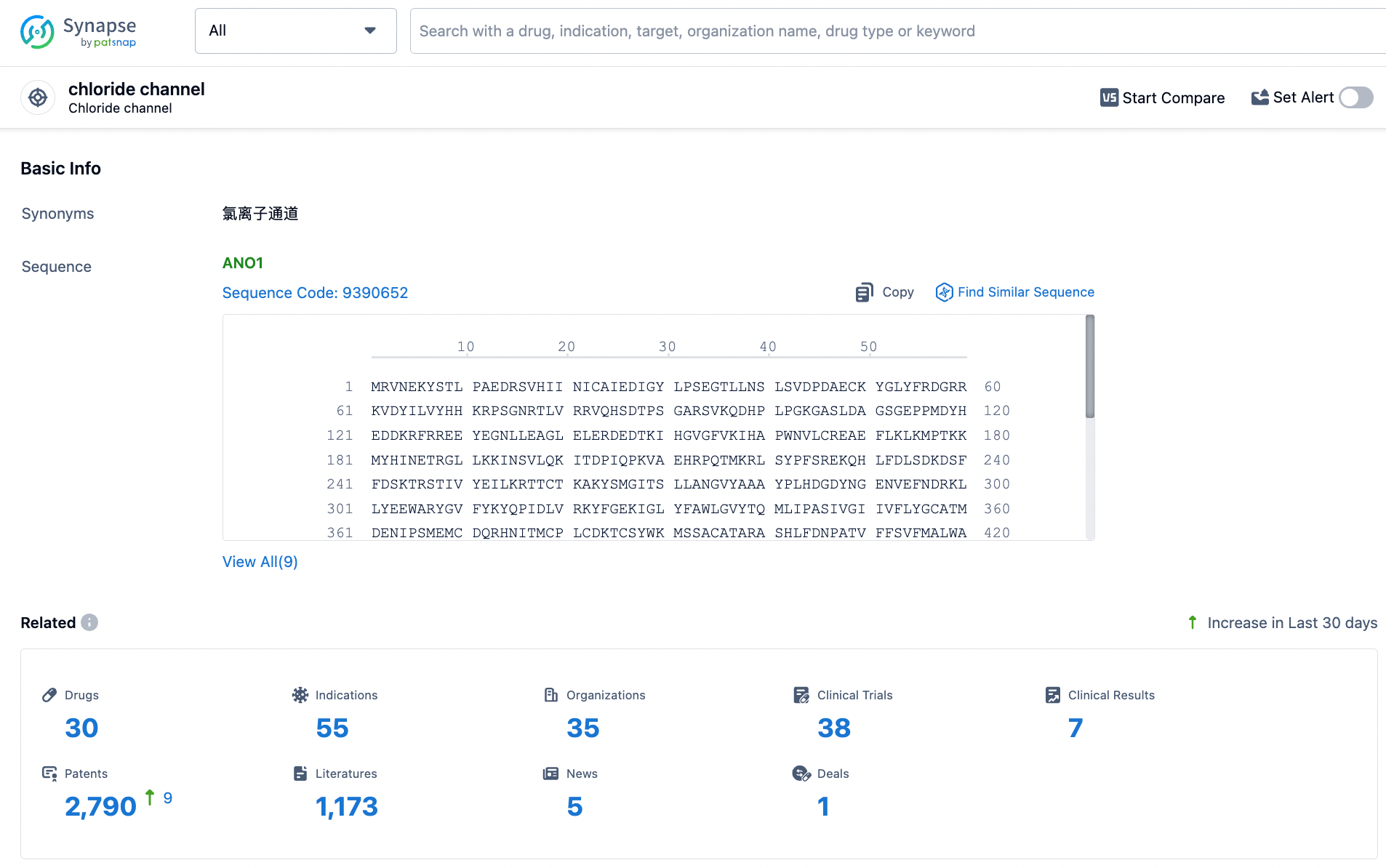
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of March 25, 2024, there are 30 investigational drugs for the chloride channel target, including 55 indications, 35 R&D institutions involved, with related clinical trials reaching 38, and as many as 2790 patents.
NMD-670 shows promise as a potential treatment for Juvenile Spinal Muscular Atrophy, targeting chloride channels in the nervous system. However, further clinical trials and regulatory approvals are necessary to determine its safety and efficacy before it can be made available to patients.