Mission Therapeutics Launches Key Study on MTX325 for Parkinson’s Disease Progression
Mission Therapeutics, an enterprise focused on clinical-phase biotechnological advancements, has initiated a Phase I human trial for MTX325. This inaugural study explores the therapeutic effects of this novel intervention aimed at mitophagy manipulation, with the goal of altering the progression of Parkinson’s Disease.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Globally, approximately 10 million individuals are battling Parkinson’s Disease, with nearly a million cases in the United States and about 1.2 million across Europe. These figures are likely to rise due to demographic shifts towards older populations.
At Mission Therapeutics, the initial group of healthy participants has been successfully administered doses as part of a segmented, flexible Phase I trial to determine the safety, tolerability, pharmacokinetic profiles, and the extent of MTX325's penetration into the brain. Plans for 2024 include to conduct dosing escalation studies in both single and multiple instances, as well as trials involving senior volunteers, followed by a special focus on Parkinson’s Disease sufferers in 2025.
Dr. Paul Thompson, PhD, the Chief Scientific Officer at Mission Therapeutics, commented: “Initiating this first-in-human trial marks a critical advancement for our company. We are eager to evaluate the efficacy of MTX325 as a drug that may alter the progression of Parkinson’s Disease. Despite current therapeutic options mitigating certain symptoms like tremors, movement rigidity, and mental difficulties, the progressive degeneration of neurons, which is fundamental to the severity of the disease, remains unaddressed.”
The commencement of this clinical study succeeded the release of a pivotal scientific article in the journal Nature Communications last December, authored by experts from Cambridge University, Harvard University, and Mission Therapeutics. The publication presented data from preclinical mouse model studies that offered considerable evidence supporting the hypothesis that MTX325 has the potential to alter Parkinson’s Disease progression by honing in on USP30.
USP30, a deubiquitylating enzyme, has been shown to obstruct mitophagy, a cellular clearance method for removing impaired mitochondria. An expanding compendium of scientific research has connected the accumulation of malfunctioning mitochondria within cells to various illnesses, which includes Parkinson’s Disease, Kidney Disease, Heart Failure, idiopathic pulmonary fibrosis, and Duchenne’s Muscular Dystrophy.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of March 25, 2024, there are 2 investigational drugs for the USP30 target, including 4 indications, 1 R&D institutions involved, and as many as 454 patents.
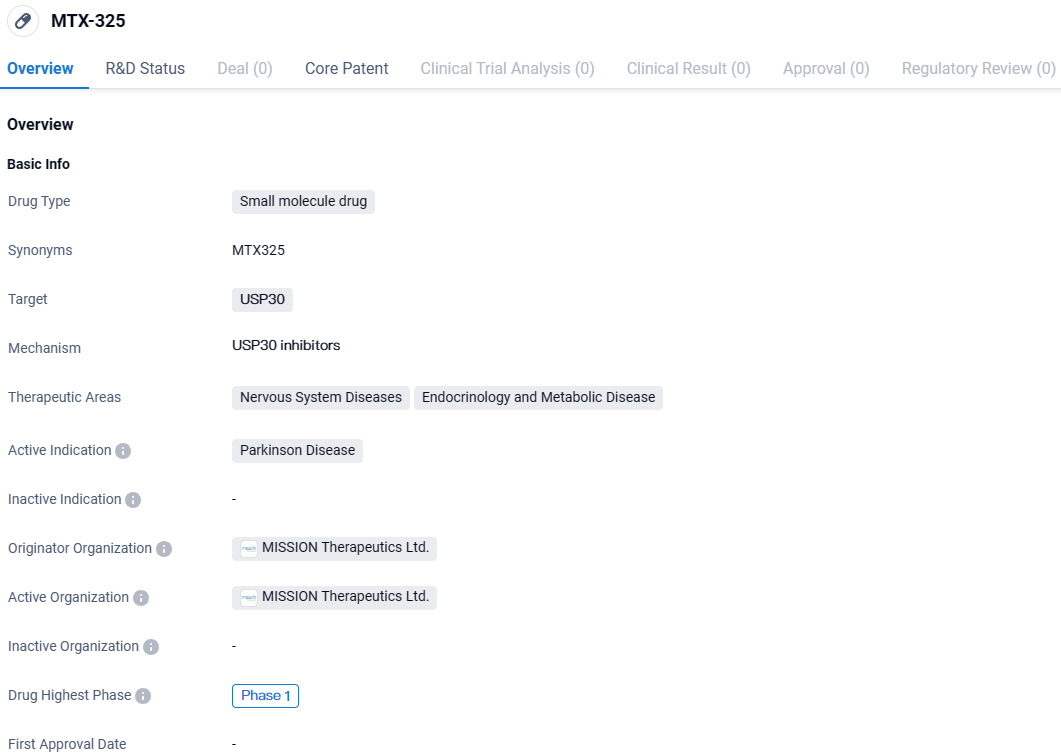
MTX-325 targets USP30 and is primarily intended for the treatment of Parkinson's Disease. The drug is currently in Phase 1 of clinical development and has potential therapeutic applications in Nervous System Diseases and Endocrinology and Metabolic Disease. Further research and clinical trials will be necessary to determine its efficacy and safety.