Fenebrutinib by Roche Significantly Reduces Disease Activity and Disability Progression in Relapsing Multiple Sclerosis Over 48 Weeks
Roche (SIX: RO, ROG; OTCQX: RHHBY) will reveal fresh 48-week results for their investigational Bruton’s tyrosine kinase (BTK) inhibitor, fenebrutinib, from the Phase II FENopta open-label extension (OLE) study at the 40th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) in Copenhagen, Denmark, on September 18, 2024. The findings suggest that patients with relapsing multiple sclerosis (RMS) who were administered fenebrutinib for up to a year showed remarkably low disease activity and did not experience any advancement in disability.
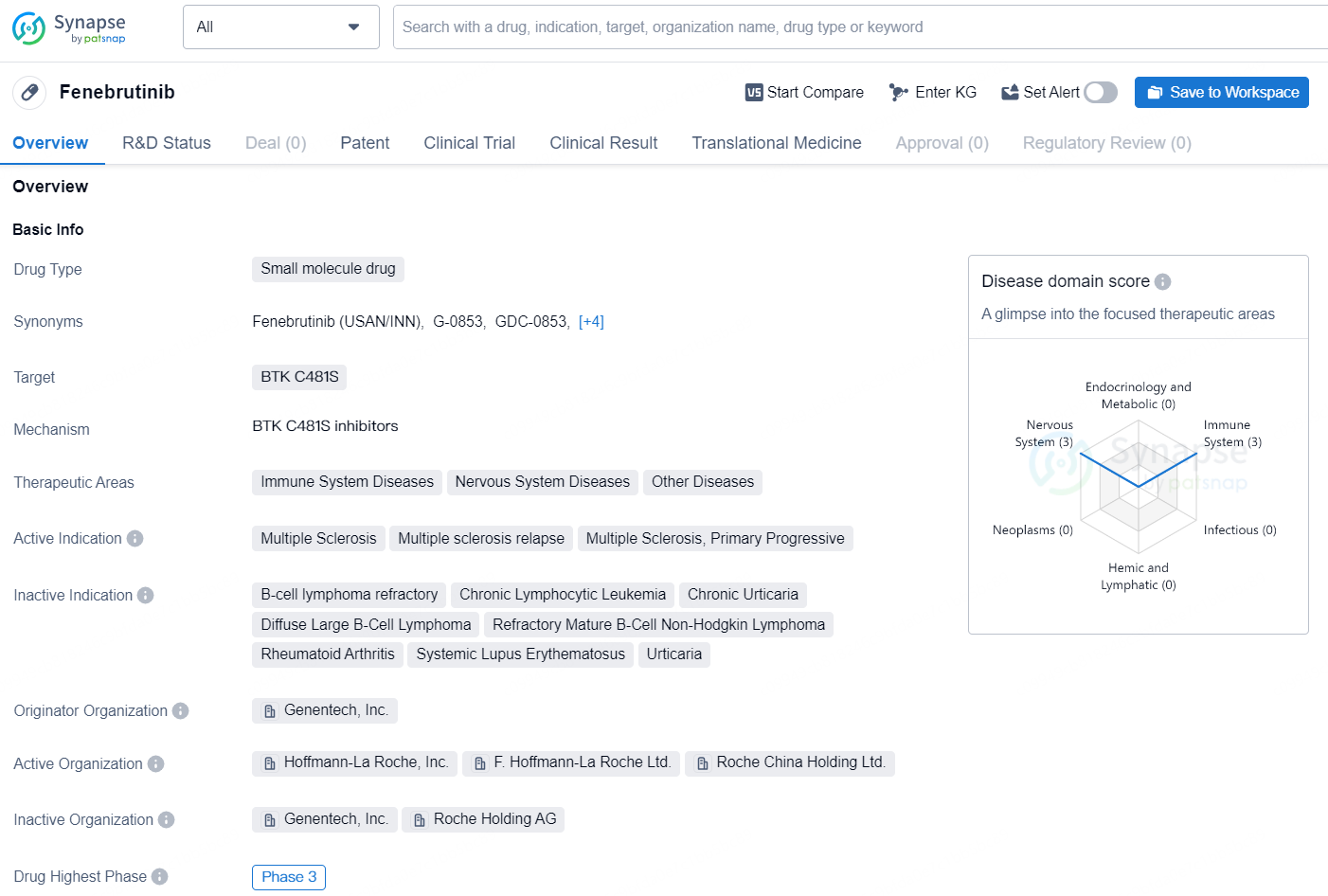
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Levi Garraway, M.D., Ph.D., Roche’s Chief Medical Officer and Head of Global Product Development, stated that over a year-long therapy, fenebrutinib, our BTK inhibitor, showed the potential to nearly eradicate disease activity and stop the advancement of disability in people with multiple sclerosis. "Should these findings be validated in the current Phase III clinical trials, fenebrutinib might substantially enhance treatment options for individuals with multiple sclerosis."
During the OLE phase, 96% of patients on fenebrutinib did not suffer relapses after one year, achieving an annualised relapse rate (ARR) of 0.04, and displayed no signs of increasing disability over a 48-week period according to the Expanded Disability Status Scale (EDSS).
Fenebrutinib significantly diminished disease activity in the brain, as confirmed by MRI imaging. After 48 weeks, 99% of the patients were devoid of T1 gadolinium-enhancing (T1-Gd+) lesions, which are markers of active inflammation. Throughout the 48-week OLE with continued fenebrutinib administration, the volume of T2 lesions, indicative of chronic disease, was reduced threefold compared to the end of the double-blind phase (-0.33 cm3 vs. -0.11 cm3, respectively).
The safety profile of fenebrutinib in the OLE was consistent with previous data. The most common adverse events (AEs) seen in more than 5% of patients included urinary tract infection (8%), COVID-19 (7%), and pharyngitis (5%). One patient (1%) experienced serious adverse events. During the OLE, one patient (1%) had a new, asymptomatic increase in alanine aminotransferase, which resolved after stopping the treatment.
Three Phase III clinical trials are ongoing, namely the FENhance 1 and 2 trials in RMS and the FENtrepid trial targeting primary progressive multiple sclerosis (PPMS). The outcomes of these studies, expected by the end of 2025, will be crucial in assessing fenebrutinib’s effect on disease progression across the multiple sclerosis spectrum.
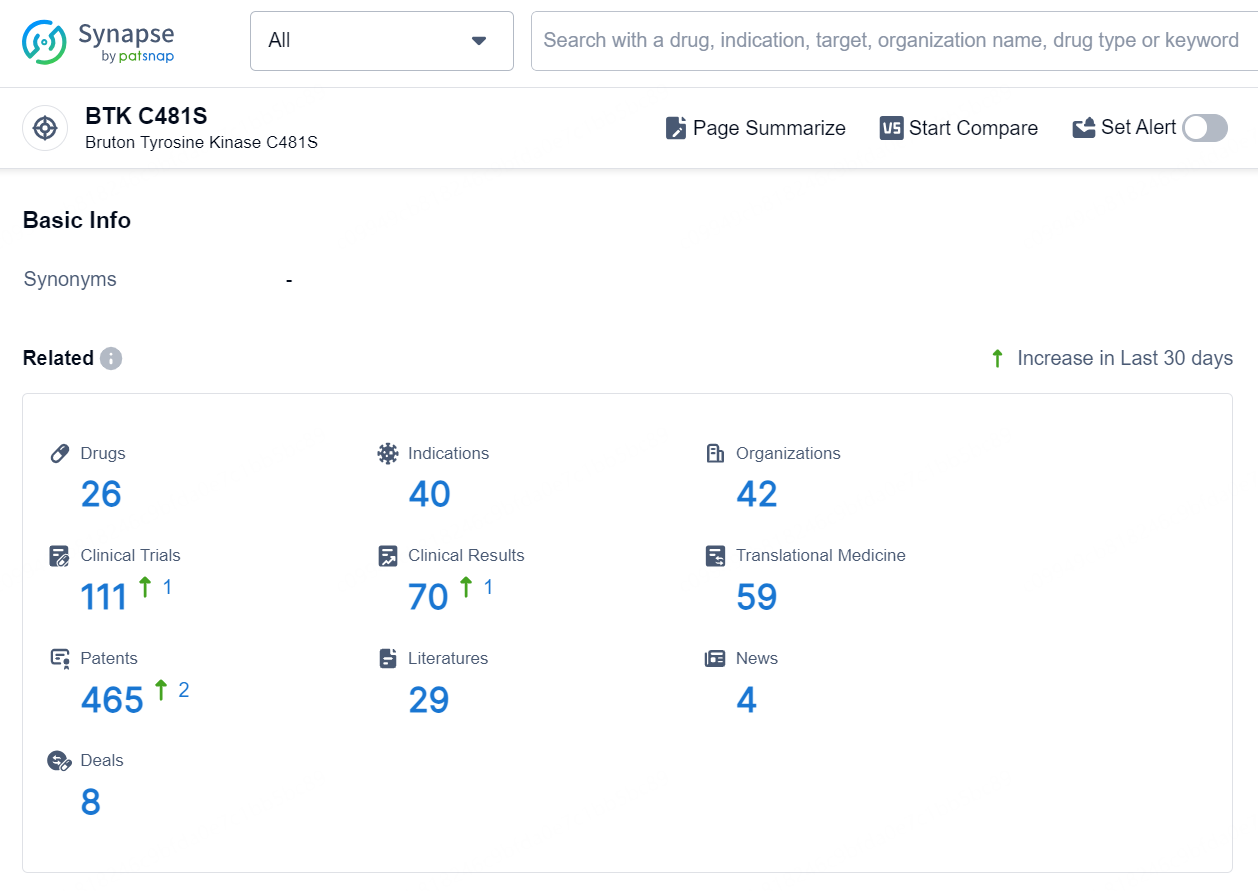
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 9, 2024, there are 26 investigational drugs for the BTK C481S targets, including 40 indications, 42 R&D institutions involved, with related clinical trials reaching 111, and as many as 465 patents.
Fenebrutinib is a small molecule drug designed to target BTK C481S, making it suitable for the treatment of immune system diseases, nervous system diseases, and other related conditions. The drug's active indication is attributed to its potential for treating multiple sclerosis, multiple sclerosis relapse, and primary progressive multiple sclerosis.