Biogen Announces Promising Results for Higher-Dose Nusinersen in SMA Therapy
Biogen Inc. (Nasdaq: BIIB) announced encouraging top-line findings from the critical segment (Part B) of the Phase 2/3 DEVOTE trial, which evaluated the safety and efficacy of a heightened dosage schedule of nusinersen for treatment-naïve, symptomatic infants with spinal muscular atrophy (SMA). The investigational higher dose regimen of nusinersen entails a faster loading schedule with two 50 mg injections administered 14 days apart, followed by an elevated maintenance dose of 28 mg every four months, in contrast to the approved SPINRAZA regimen. The trial reached its primary endpoint at the six-month milestone, showing a statistically significant improvement in motor function in infants receiving the higher dose regimen compared to a predefined matched sham control group from the ENDEAR study.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Significant progress has been achieved in SMA treatment, yet many needs remain unmet. Drawing upon the established profile of SPINRAZA, which has been developed over the last ten years, we continually seek ways to enhance the efficacy without compromising safety," stated Stephanie Fradette, Pharm.D., Biogen's Head of Neuromuscular Development. "Encouraging topline results from the DEVOTE study suggest that a higher dosage can more rapidly slow neurodegeneration, evidenced by greater reductions in neurofilament levels at day 64 compared to the approved dose. Over the long term, this higher dosage demonstrated meaningful clinical benefits for infants with symptomatic SMA. We are keen to share detailed findings with the SMA community and health authorities."
DEVOTE is a tripartite study that involved 145 participants of various ages and SMA subtypes. The critical Part B cohort included treatment-naïve children with infantile-onset SMA (n=75), who were randomized in a 2:1 ratio to receive either the investigational higher dose regimen of nusinersen or the approved 12 mg regimen (comprising four loading doses followed by maintenance doses every four months). The primary endpoint of Part B measured the change from baseline on the Children's Hospital of Philadelphia-Infant Test of Neuromuscular Disorders (CHOP-INTEND) at six months, comparing the higher dose regimen of nusinersen against a matched, untreated sham control group from the Phase 3 ENDEAR study. ENDEAR was one of the pivotal studies that supported the regulatory approval of SPINRAZA® 12 mg.
The cohort receiving the higher dose exhibited a statistically significant improvement compared to the matched sham control on the primary endpoint, which assessed the change in CHOP-INTEND from baseline to six months (least squares mean difference: 26.19; p<0.0001). Results indicated a preference for the higher dose regimen over sham across secondary endpoints and suggested a trend favoring the higher dose regimen over the current 12 mg regimen on key biomarkers and efficacy measures. The higher dose regimen was generally well-tolerated, with adverse events consistent with those seen in SMA and the known safety profile of nusinersen. The proportion of serious adverse events was lower in the higher dose group (60%; 30) compared to the 12 mg group (72%; 18). Detailed results from DEVOTE will be presented at upcoming medical conferences.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
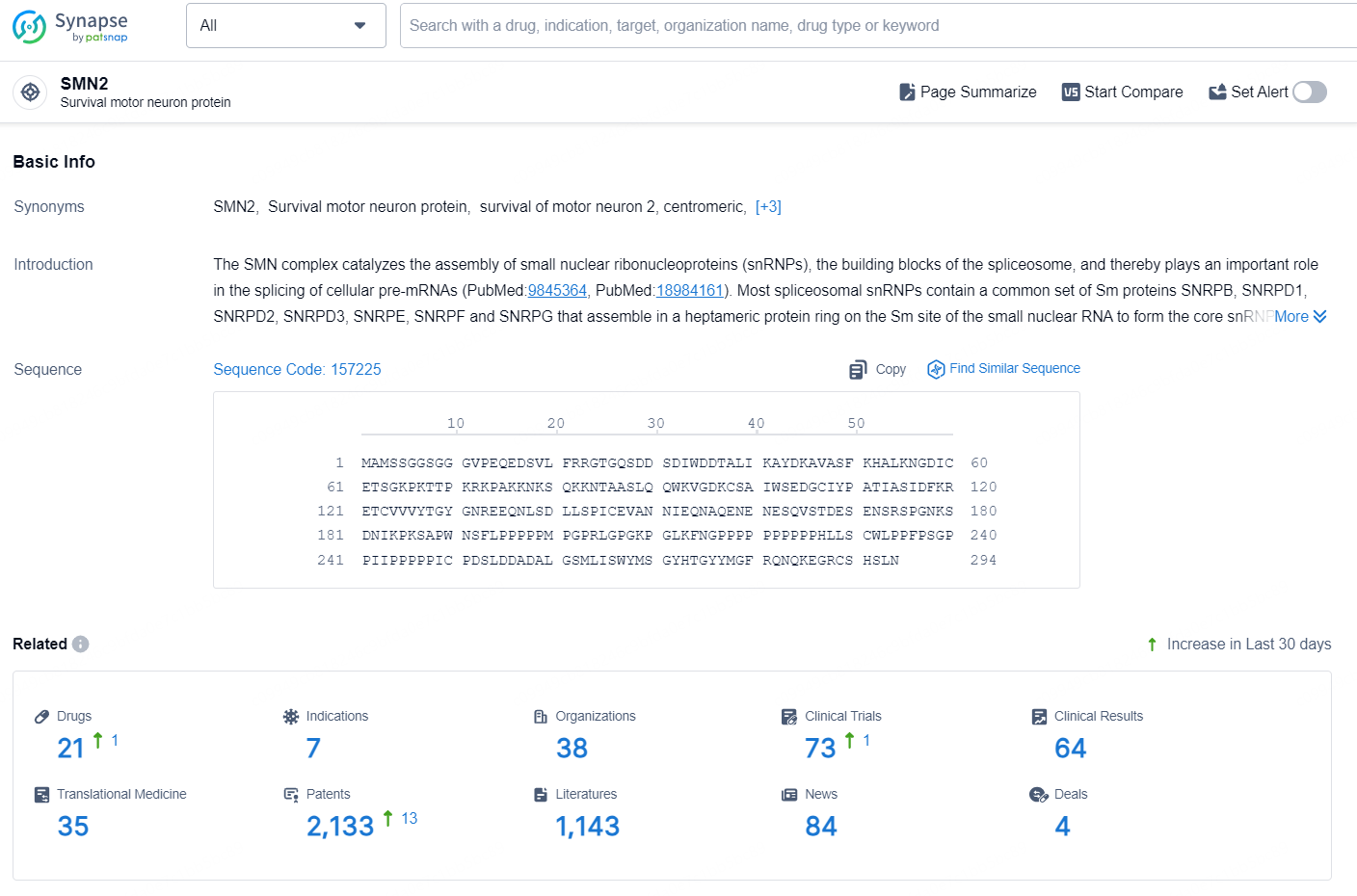
According to the data provided by the Synapse Database, As of September 9, 2024, there are 21 investigational drugs for the SMN2 target, including 7 indications, 38 R&D institutions involved, with related clinical trials reaching 73, and as many as 2133 patents.
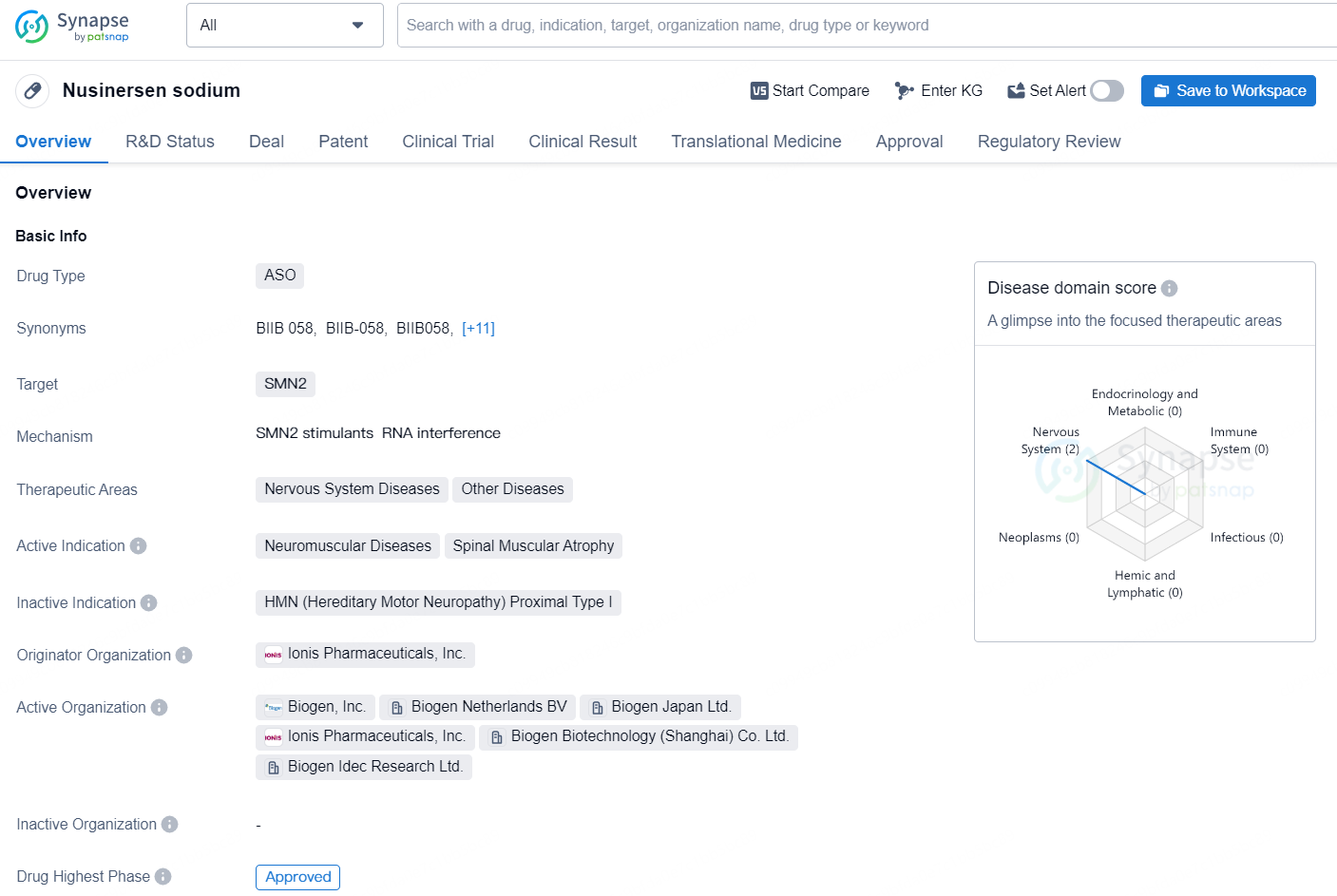
Nusinersen sodium is an antisense oligonucleotide (ASO) drug that targets SMN2, and is mainly used for treating neuromuscular diseases and spinal muscular atrophy. It falls under the therapeutic areas of Nervous System Diseases and Other Diseases. The originator organization of Nusinersen sodium is Ionis Pharmaceuticals, Inc. The drug has reached the highest phase of approval both globally and in China. Its first approval date globally was in December 2016, with the United States being the first country/location to approve it. Nusinersen sodium is regulated under Overseas New Drugs Urgently Needed in Clinical Settings, Fast Track, and Orphan Drug categories.