First Patient Treated in Phanes Therapeutics' PT886 and KEYTRUDA® Trial
Phanes Therapeutics, Inc. (Phanes), a biotechnology firm in the clinical development phase dedicated to cutting-edge drug discovery and development for cancer treatment, declared that the initial patient has received treatment in the clinical trial evaluating PT886 in conjunction with Merck’s anti-PD-1 agent, KEYTRUDA® (pembrolizumab). This administration took place within a group of patients with gastric and gastroesophageal junction malignancies.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
As announced previously, Phanes is undertaking this research through a clinical trial collaboration with Merck (referred to as MSD outside the United States and Canada). PT886 is an innovative native IgG-like bispecific antibody (bsAb) designed to target claudin 18.2 and CD47. In 2022, it received orphan drug designation (ODD) from the FDA for pancreatic cancer treatment and was awarded Fast Track designation this year for patients with metastatic claudin 18.2-positive pancreatic adenocarcinoma. PT886 is currently being explored both as a standalone treatment and in combination with pembrolizumab, or with chemotherapy, whether administered separately or alongside pembrolizumab.
The TWINPEAK study, a multi-center Phase I/II clinical trial (NCT05482893), is presently assessing the safety, tolerability, pharmacokinetics, pharmacodynamics, and initial efficacy of PT886 in individuals with locally advanced or metastatic gastric, gastroesophageal junction, and pancreatic cancers. Additionally, a Phase I clinical trial for PT886 is underway in China (CTR20241655).
KEYTRUDA® is a trademark owned by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., located in Rahway, NJ, USA.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
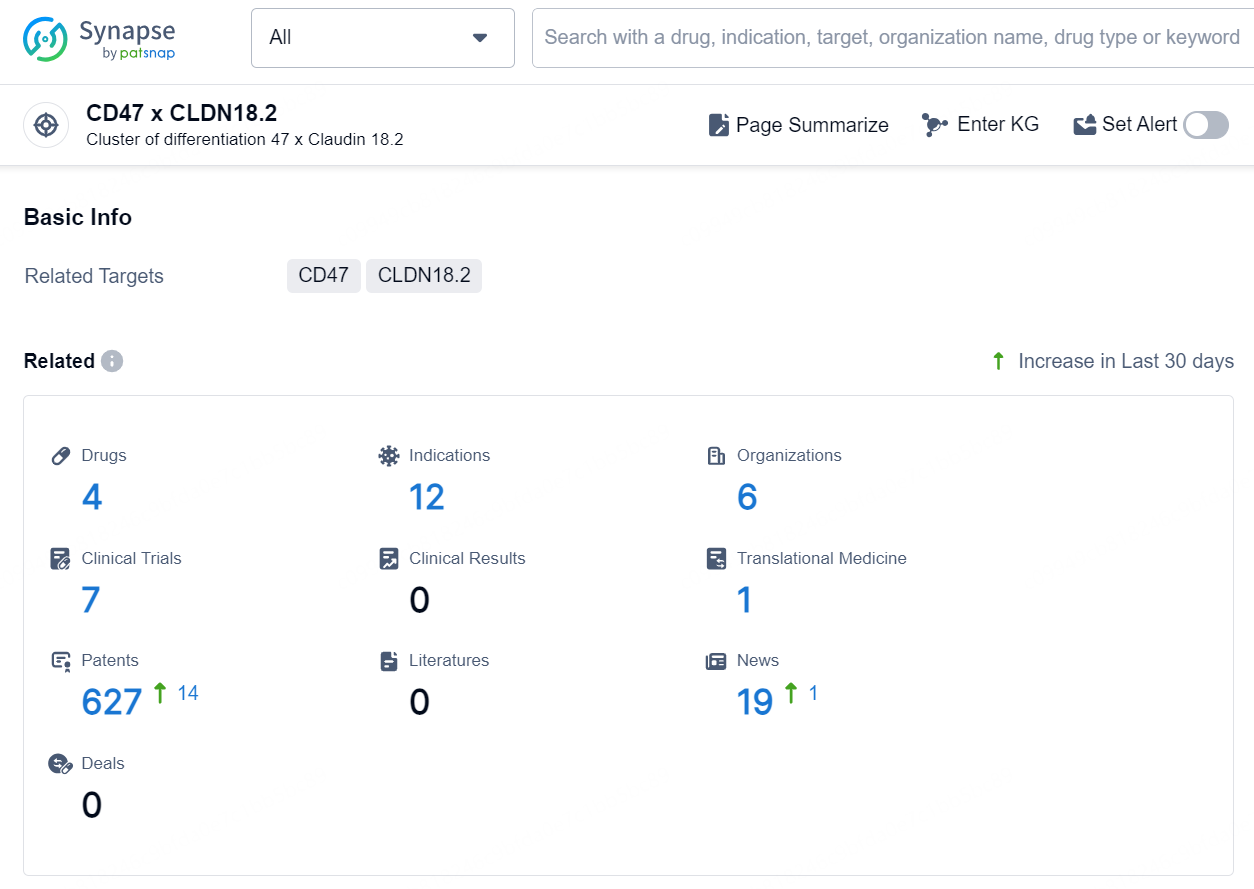
According to the data provided by the Synapse Database, As of October 11, 2024, there are 4 investigational drug for the CD47 x CLDN18.2 targets, including 12 indications, 6 R&D institutions involved, with related clinical trials reaching 7, and as many as 627 patents.
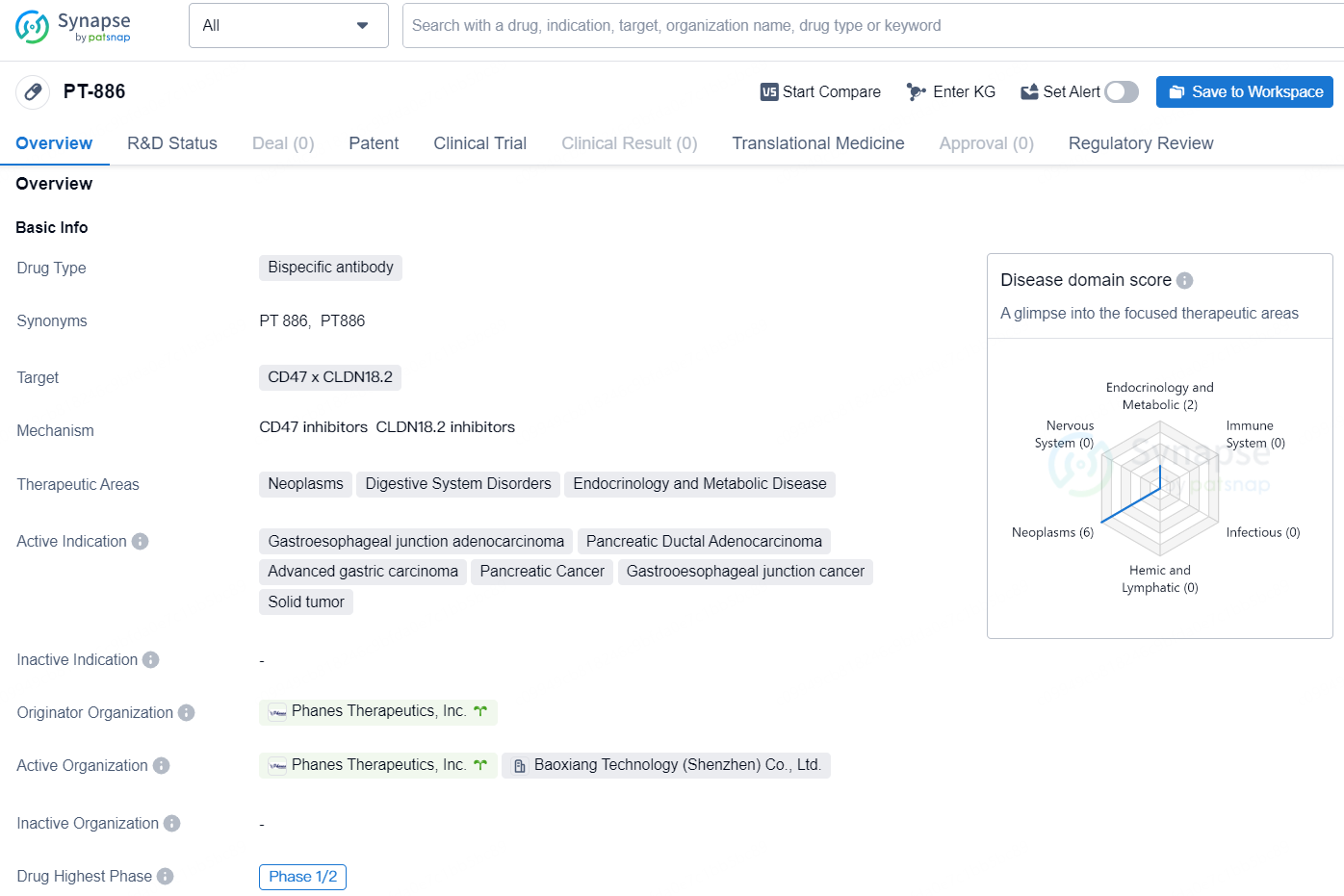
The drug PT-886 is a bispecific antibody that targets CD47 x CLDN18.2 and is being developed by Phanes Therapeutics, Inc. for the treatment of various neoplastic and digestive system disorders, as well as endocrinology and metabolic diseases. The drug is currently in the highest phase of development, which is Phase 1/2 globally and Phase 1 in China.