First Wave BioPharma: Focused on Targeted Oral Therapies for Gastrointestinal Diseases
On March 14th, First Wave BioPharma, a company focused on targeted oral therapies for gastrointestinal diseases, announced the completion of an all-stock transaction to acquire ImmunogenX. Following the merger, First Wave will concentrate on advancing a portfolio of gastrointestinal disease therapies, including latiglutenase, which encompasses multiples late-stage clinical assets.
After the acquisition, the company issued 365,162 ordinary shares to the shareholders of ImmunogenX, representing 19.02% of the currently issued and outstanding shares, along with 11,777,418 newly issued Series G convertible preferred shares. The post-merger fully diluted equity value is estimated to be $104 million.
Following the acquisition, the main pipeline arrangements for First Wave are as follows:
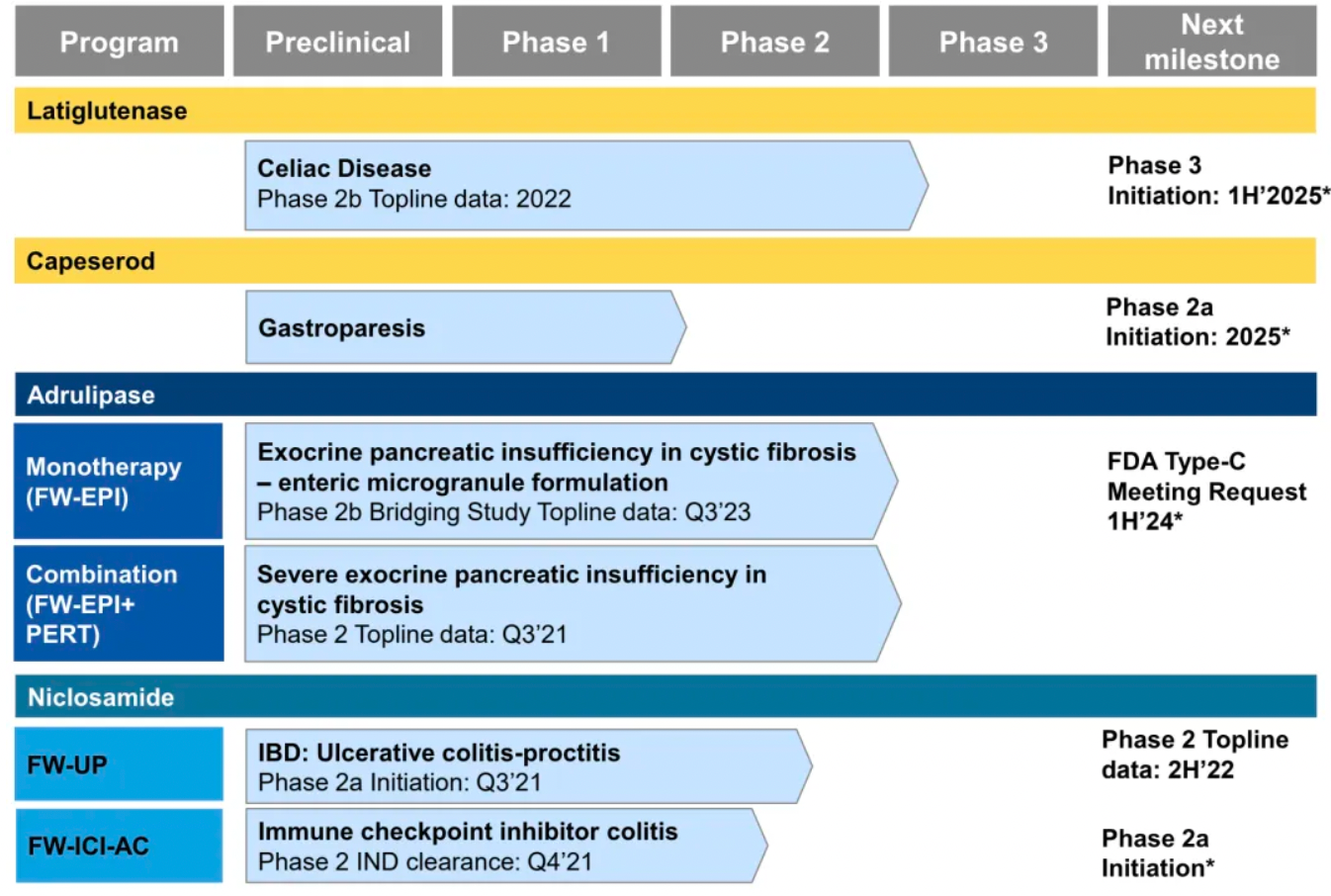
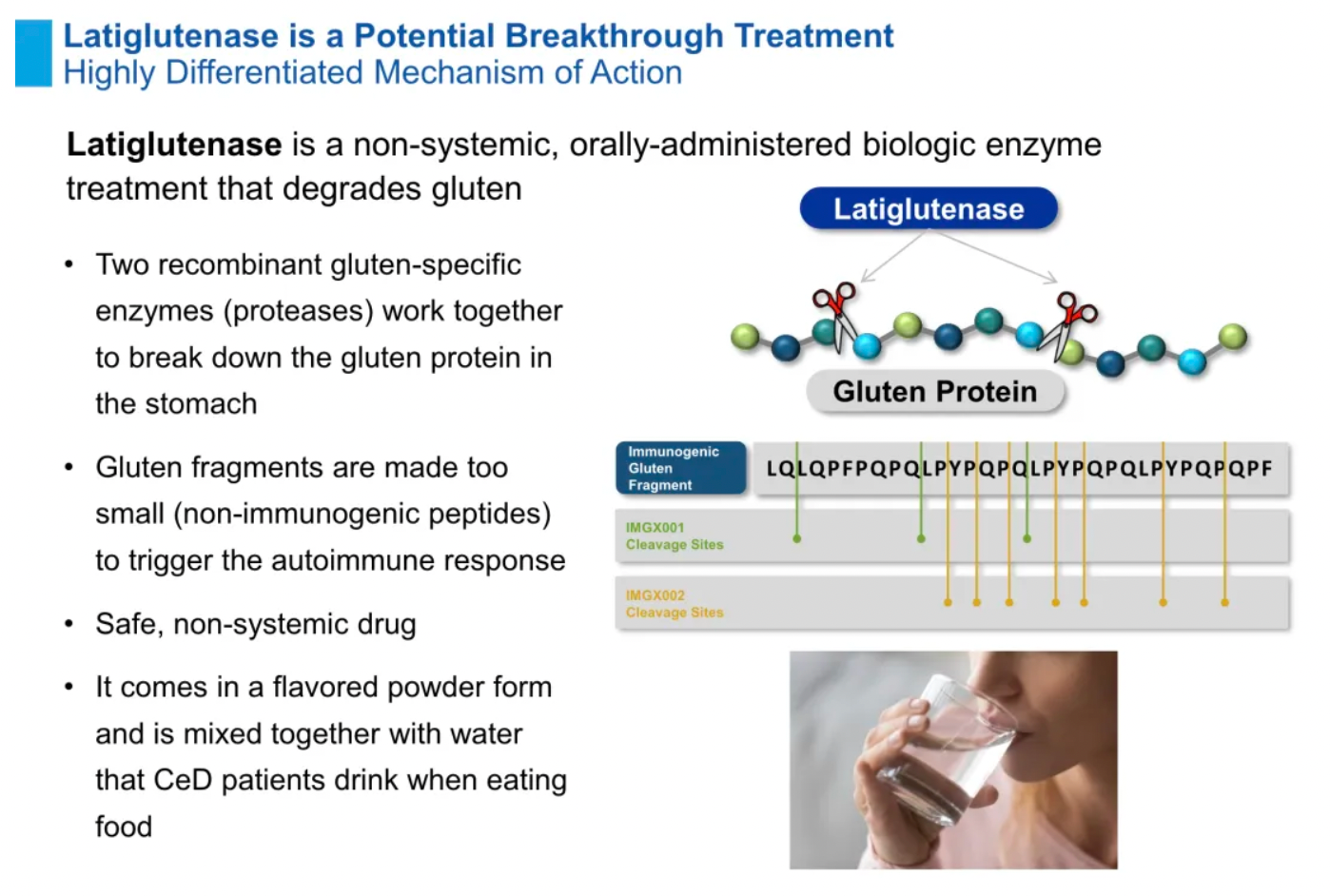
Latiglutenase: This is a potential first-in-class oral biologic therapy designed to aid in the digestion of gluten and possibly alleviate the symptoms of celiac disease. Celiac disease is an autoimmune disorder characterized by intolerance to gluten, a protein found in wheat, barley, and rye. The development of latiglutenase offers new therapeutic hope for patients affected by this condition.

Capeserod: This is a selective partial agonist for the 5-HT4 receptor, currently under development for the treatment of gastroparesis. Gastroparesis is a condition characterized by delayed gastric emptying, which can lead to symptoms such as nausea, vomiting, and a feeling of fullness. The mechanism of action of capeserod is aimed at improving the motility function of the gastrointestinal tract.

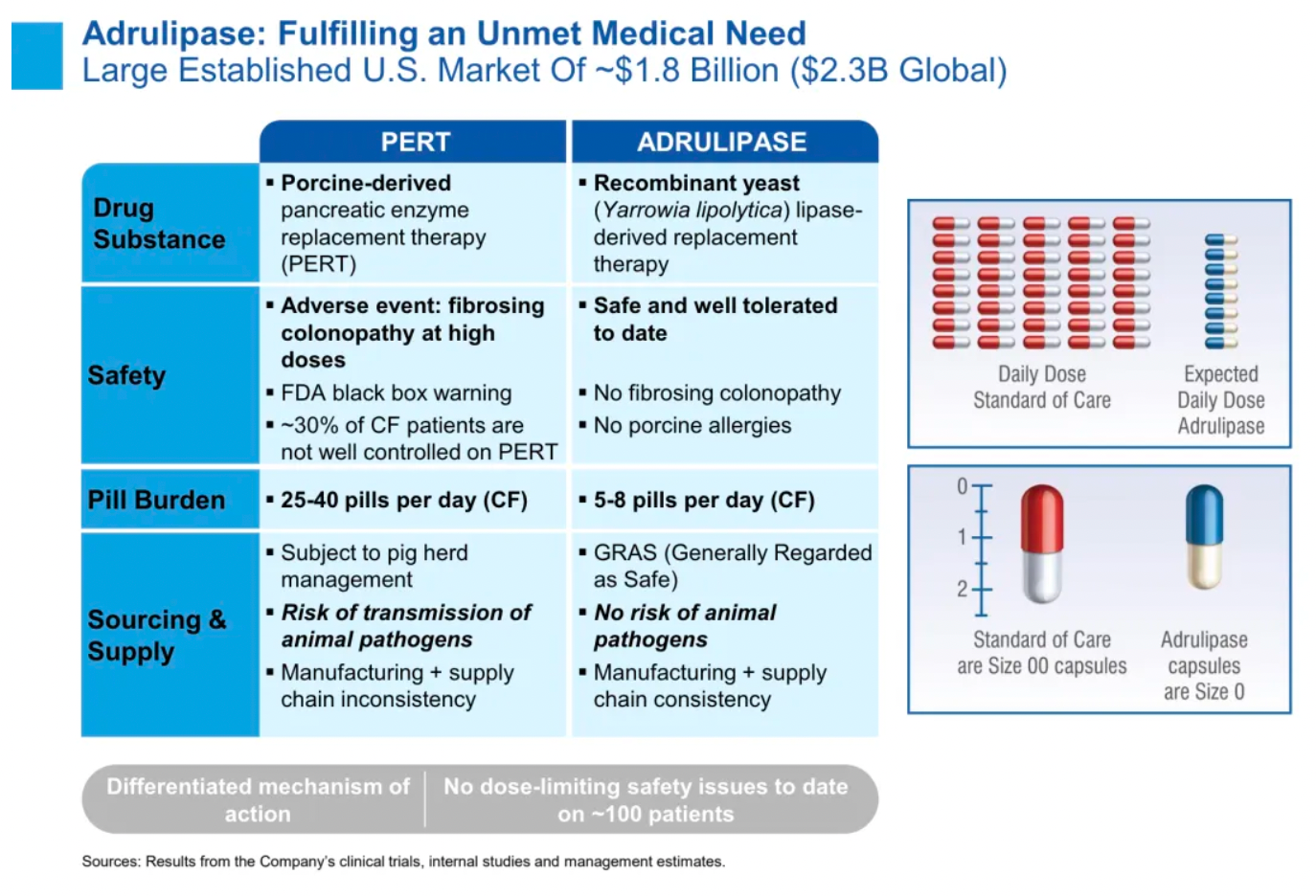
Adrulipase: This is a recombinant lipase designed to aid in the digestion of fats and other nutrients for patients with exocrine pancreatic insufficiency. Patients with this condition are unable to produce sufficient digestive enzymes from the pancreas, leading to ineffective absorption of fats, proteins, and other nutrients from food. The use of adrulipase aims to improve their nutritional absorption.
Niclosamide: This drug was approved by the U.S. FDA in 1982 for the treatment of intestinal tapeworm infections. Niclosamide has shown potential as a non-steroidal anti-inflammatory therapy for treating mild to moderate inflammatory bowel disease (IBD). First Wave has conducted several Phase II clinical trials on niclosamide, including for the treatment of ulcerative proctitis/proctosigmoiditis (the most common form of ulcerative colitis). The company also holds an open Investigational New Drug (IND) application for treating colitis and diarrhea in patients with metastatic cancer undergoing treatment with immune checkpoint inhibitors.

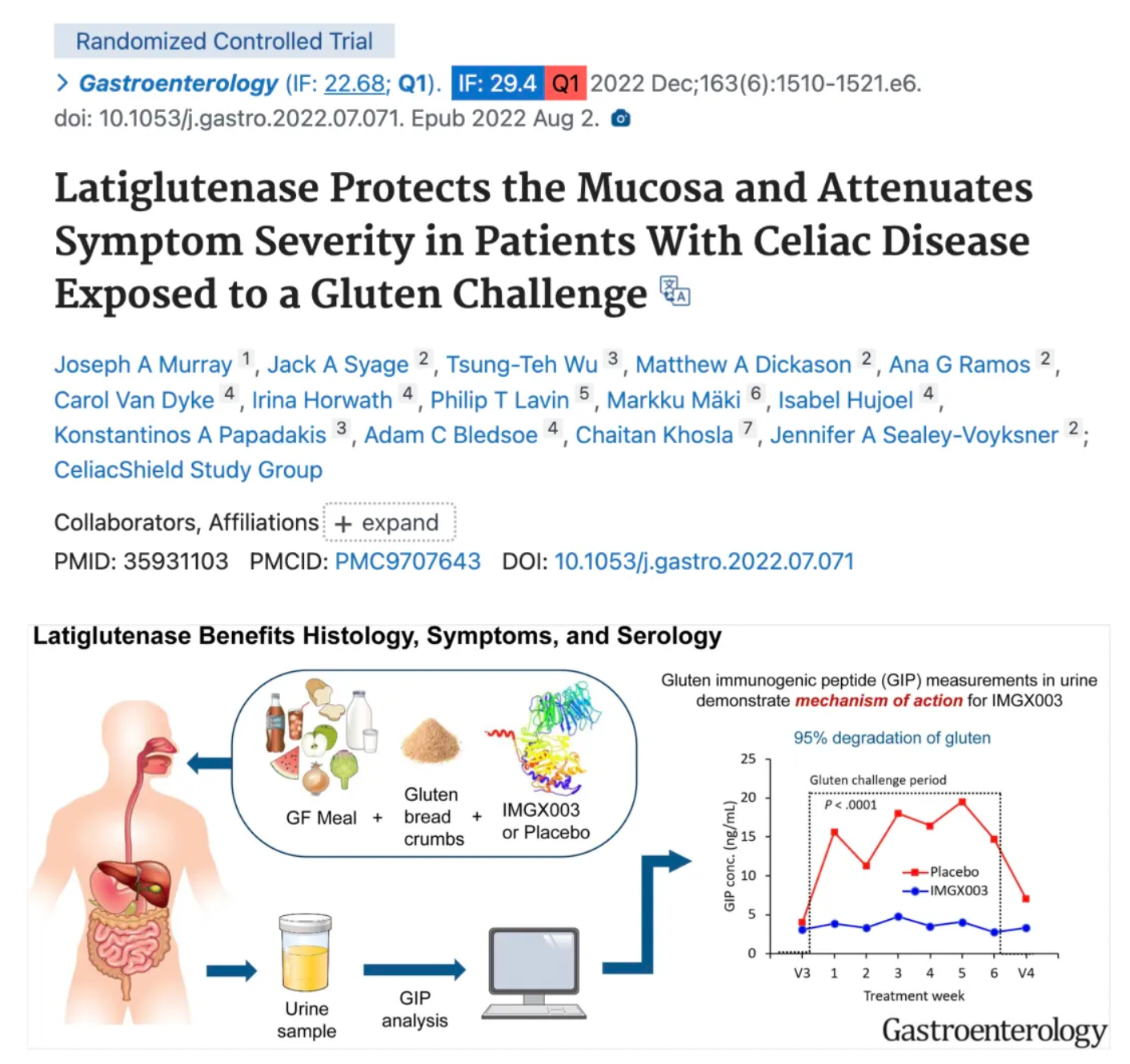
According to reports, latiglutenase (IMGX-003) demonstrated effectiveness in reducing intestinal damage and alleviating symptoms of celiac disease in two phase II trials involving approximately 200 patients. The phase III clinical program for latiglutenase has been reviewed by the Gastrointestinal Disorders Division of the U.S. Food and Drug Administration (FDA), and the clinical trial is expected to commence in early 2025.
Capeserod is a selective partial agonist of the 5-hydroxytryptamine 4 (5-HT4) receptor, originally developed by Sanofi for research in the treatment of neurological disorders. The 5-HT4 receptors also play a significant role in the human digestive system, where they are involved in regulating gastrointestinal motility and sensory functions, and are crucial for maintaining normal gastrointestinal rhythm and promoting gastric emptying. Theoretically, by activating these receptors, Capeserod could enhance the motility frequency and strength of the gastrointestinal tract, thus improving symptoms such as chronic idiopathic constipation and gastroparesis. It was once researched as a potential treatment for these diseases, especially those associated with insufficient gastrointestinal motility.
Approximately twenty years ago, Sanofi's phase II clinical trial of Capeserod for Alzheimer's disease and urinary incontinence was halted. First Wave, utilizing AI-based analytical tools, supported the potential application of Capeserod's mechanism in treating multiple gastrointestinal diseases, areas characterized by large markets and significant unmet medical needs. In September 2023, First Wave entered into a transfer agreement with Sanofi to reinitiate the research and development of Capeserod for gastrointestinal diseases, considering advancing it to clinical trial stages to verify its safety and effectiveness for new indications.
For more information about First Wave BioPharma, please click the image below to access the Synapse database.




