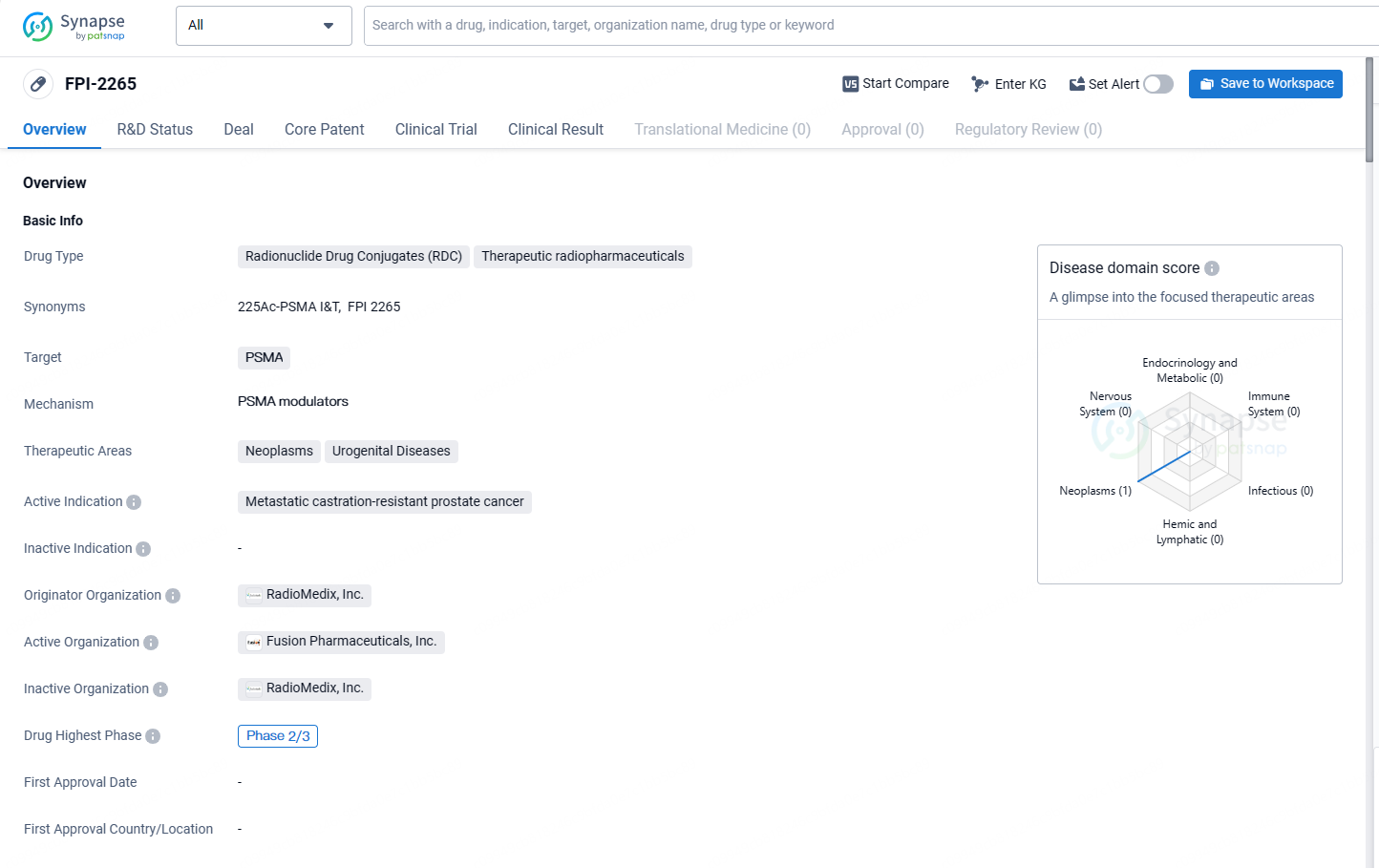
Fusion Pharmaceuticals Initiates Phase 2 AlphaBreak Trial of FPI-2265 in Metastatic Prostate Cancer
Fusion Pharmaceuticals Inc., a firm in the clinical phase specializing in oncology, recently disclosed the commencement of patient dosing in the Phase 2 segment of the AlphaBreak study. This trial is assessing the efficacy of FPI-2265 (225Ac-PSMA I&T) in individuals diagnosed with metastatic castration-resistant prostate cancer. Fusion Pharmaceuticals focuses on crafting cutting-edge radioconjugates for use as targeted therapies.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Chief Medical Officer Dmitri Bobilev, M.D. of Fusion stated, "The recent Phase 2 TATCIST trial results presented at the AACR Annual Meeting confirm the safety and clinical effectiveness of Actinium-based PSMA targeting RCs, including Fusion's FPI-2265. We are optimistic about FPI-2265 as a significant emerging therapy for mCRPC patients and are enthusiastic about the progression to the AlphaBreak trial."
XCancer's CEO and investigator, Luke Nordquist, M.D., FACP, added, "Many mCRPC patients still require new therapeutic alternatives particularly after advancing following lutetium-based radiotherapy treatments. The clinical outcomes and safety observed with FPI-2265 are promising. Starting the AlphaBreak trial represents progress towards filling the treatment void for these patients."
The AlphaBreak trial, a Phase 2/3, randomized, open-label, multicenter study, investigates the safety and effectiveness of FPI-2265 in mCRPC patients previously treated with 177Lu-PSMA radiotherapy. The study includes a Phase 2 segment focusing on dose optimization, examining the possible safety and efficacy enhancements of two new dosing schedules compared to the prior 100 kBq/kg every eight weeks regimen.
Enrollment for the Phase 2 segment of the AlphaBreak trial will likely close by the end of 2024 with around 60 participants. Upon completing this phase and holding a conclusive meeting with the FDA, the trial will transition into the Phase 3 global registration phase. This next phase, projected to start in 2025, plans to enroll about 550 patients.
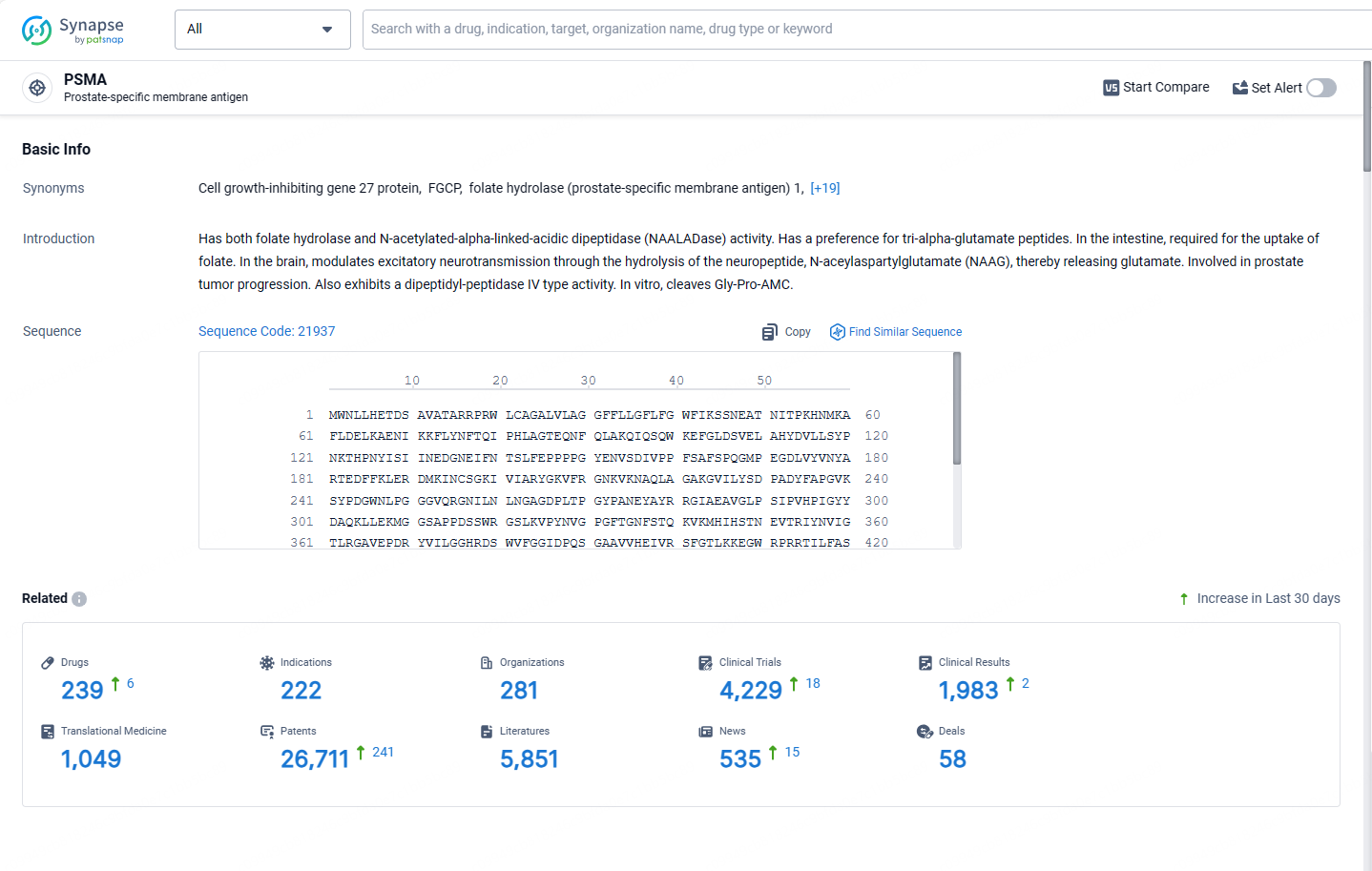
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of May 13, 2024, there are 239 investigational drugs for the PSMA targets, including 222 indications, 281 R&D institutions involved, with related clinical trials reaching 4229, and as many as 26711 patents.
FPI-2265 is an actinium-225 based PSMA targeting RC, for mCRPC, currently in a Phase 2 trial. Actinium-225 emits alpha particles and holds the promise of being a next-generation radioisotope in cancer treatment. Currently, FPI-2265 is in the highest phase of clinical development, which is Phase 2/3. Phase 2/3 trials typically involve a significant number of participants and aim to further assess the drug's effectiveness and potential side effects.