Foghorn Therapeutics reports the dosing of its first patient in a Phase 1 trial of FHD-909
Foghorn® Therapeutics Inc. (Nasdaq: FHTX), a biotechnology firm in the clinical development stage, is at the forefront of creating a new category of therapies aimed at addressing serious illnesses by rectifying abnormal gene expression. The company has now reported that the initial patient has received the FHD-909 (LY4050784) dosage as part of the Phase 1 clinical trial targeting cancers with SMARCA4 (BRG1) mutations, focusing primarily on non-small cell lung cancer (NSCLC) as the main patient group. FHD-909 is an innovative oral compound known for its potency and exceptional selectivity for SMARCA2 (BRM), particularly in contrast to the closely related protein SMARCA4.
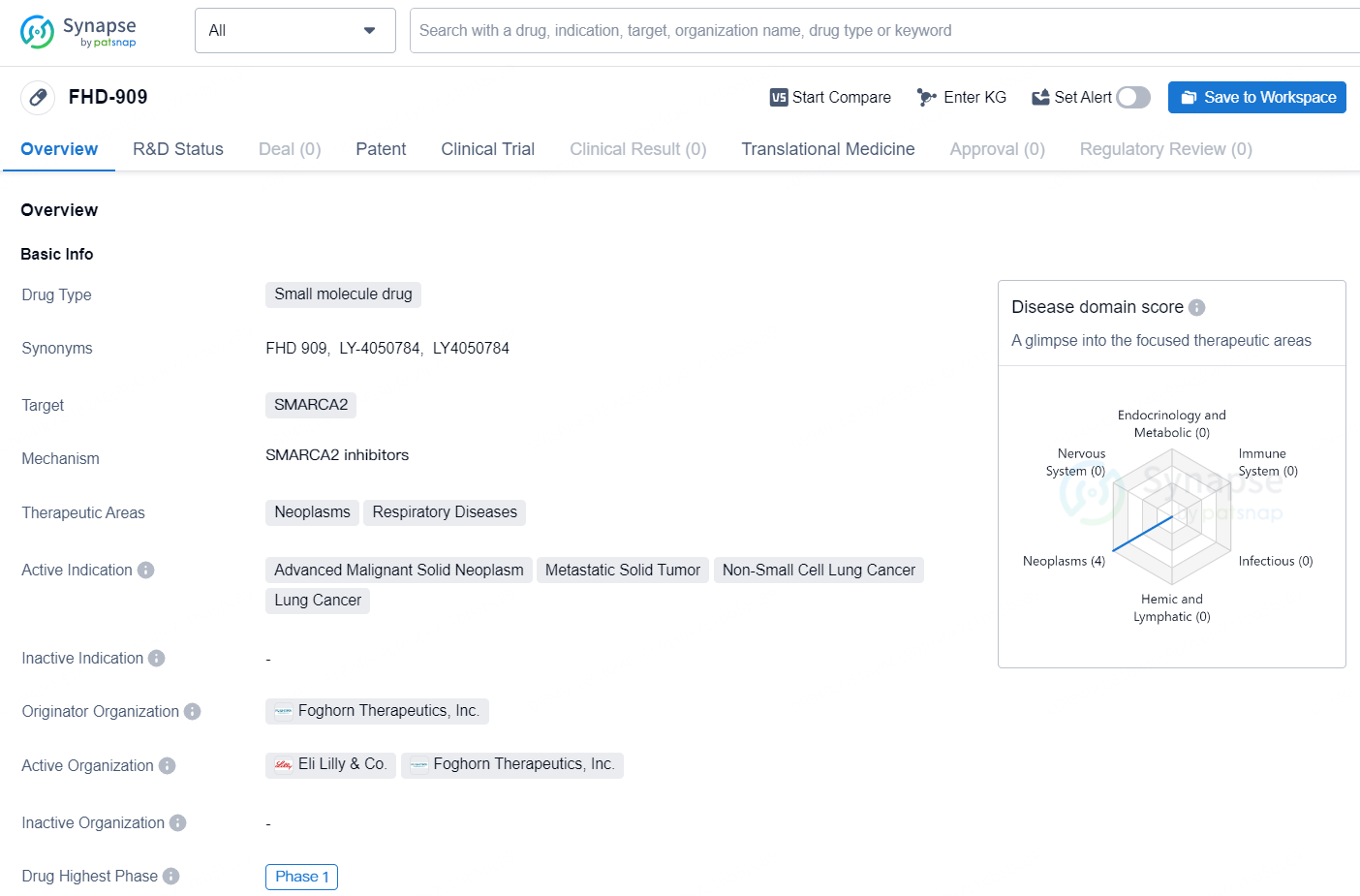
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
FHD-909 represents the inaugural SMARCA2 selective inhibitor to progress into clinical trials. "Dosing the first participant signifies a key achievement for the initiative and our partnership with Lilly," stated Adrian Gottschalk, President and CEO of Foghorn. "FHD-909 exhibits considerable selectivity over the closely related SMARCA4, providing a promising synthetic lethality approach for common SMARCA4 mutations and their susceptibility to SMARCA2 inhibition in non-small cell lung cancer (NSCLC) and various other solid tumors. We anticipate further development of FHD-909 alongside Lilly."
A Phase 1 open-label, multicenter study will evaluate the safety, tolerability, and preliminary efficacy of FHD-909 in individuals with locally advanced or metastatic solid tumors featuring a SMARCA4 alteration.
Preclinical trials indicate that FHD-909 is a powerful and selective SMARCA2 inhibitor showing strong anti-tumor monotherapy capabilities. In vivo studies have revealed that FHD-909 is well-tolerated, demonstrating dose-dependent regulation of SMARCA2 target genes, along with notable dose-dependent tumor growth suppression and regression in SMARCA2 mutant xenograft mouse models.
In December 2021, Foghorn revealed a strategic partnership with Lilly to develop innovative oncology treatments. This collaboration includes a 50/50 U.S. co-development and co-commercialization agreement for Foghorn’s Selective SMARCA2 oncology initiative, as well as another undisclosed oncology target. Additionally, the partnership encompasses three discovery projects utilizing Foghorn’s proprietary Gene Traffic Control® platform.
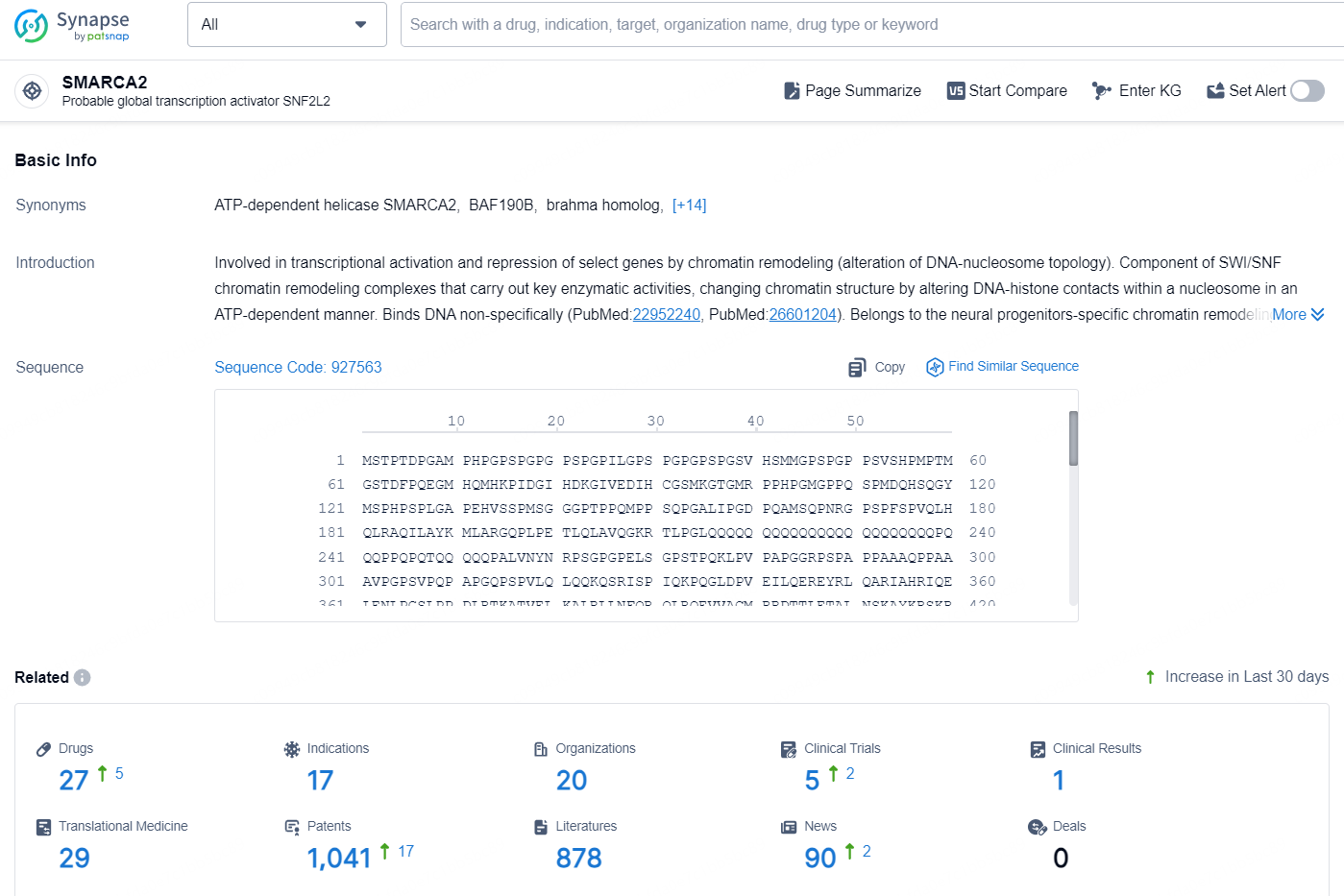
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 10, 2024, there are 27 investigational drugs for the SMARCA2 targets, including 17 indications, 20 R&D institutions involved, with related clinical trials reaching 5, and as many as 1041 patents.
FHD-909 is a small molecule drug developed by Foghorn Therapeutics, Inc. The drug targets the SMARCA2 gene and is intended for the treatment of neoplasms and respiratory diseases. FHD-909 is specifically designed for use in the treatment of advanced malignant solid neoplasms, metastatic solid tumors, non-small cell lung cancer, and lung cancer.