Xencor Presents Initial Research on XmAb942, a Durable Anti-TL1A Antibody for Inflammatory Bowel Disease Treatment
Xencor, Inc. (NASDAQ: XNCR), a biopharmaceutical firm focused on clinical development of engineered antibodies for treating cancer and various critical illnesses, announced the publication of preclinical findings on XmAb942. This data will be showcased in a poster presentation during the United European Gastroenterology (UEG) Week, set to take place on Tuesday, October 15 in Vienna, Austria.
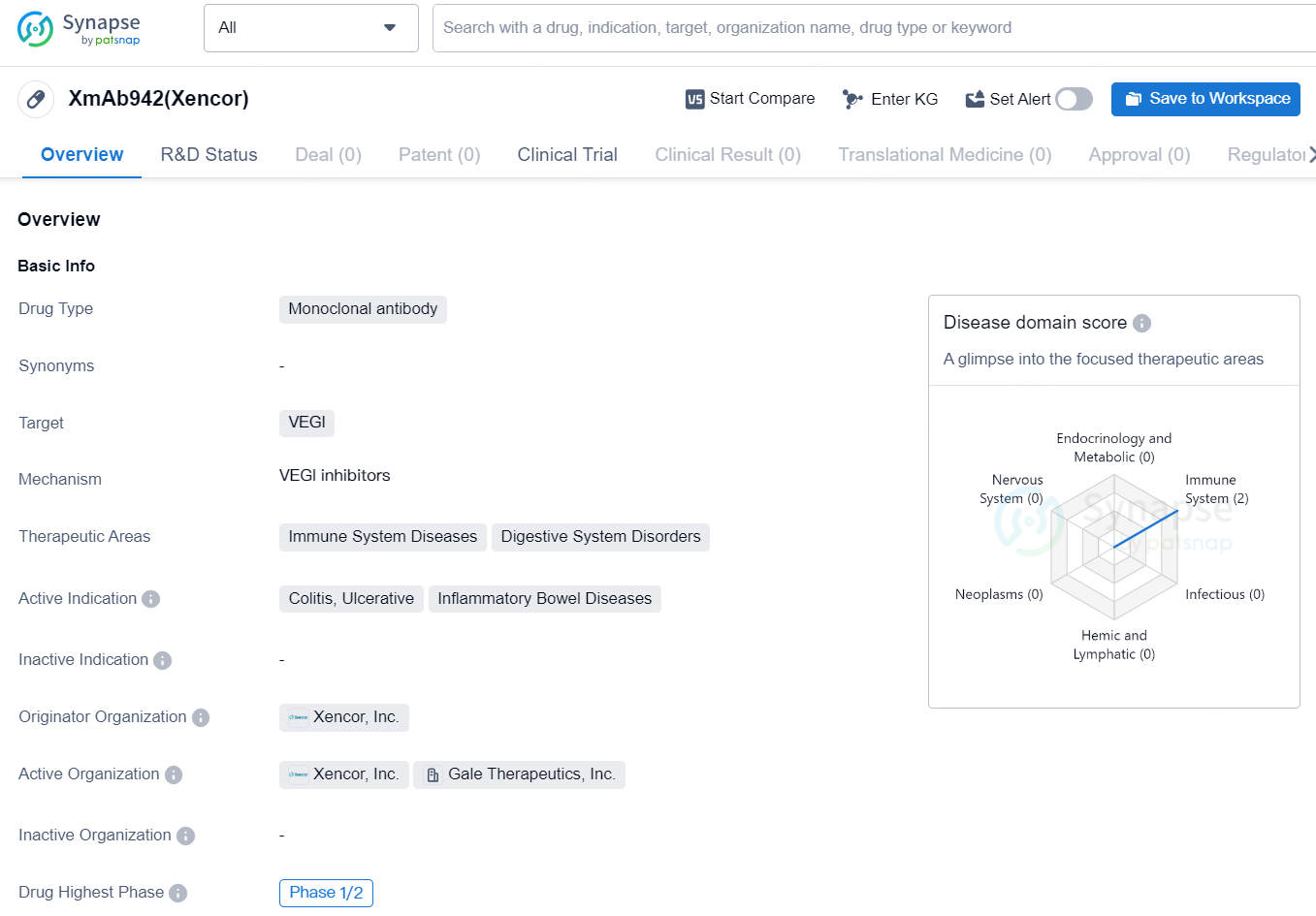
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
XmAb942 is a potent investigational anti-TL1A antibody with an extended half-life, being developed for individuals suffering from inflammatory bowel diseases (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD). The initial iterations of anti-TL1A antibodies were formulated to inhibit the interaction between the DR3 receptor and the ligand TL1A, resulting in decreased disease severity in patients with UC and CD, as evidenced in several clinical trials. Xencor plans to initiate dosing in the first human trial of XmAb942 in healthy participants in the fourth quarter of 2024 and aims to report preliminary findings from the single-ascending dose segment of the trial in the first half of 2025.
“Our objective is for XmAb942 to emerge as a potential best-in-class, next-generation anti-TL1A antibody that offers superior potency and less frequent dosing compared to first-generation TL1A-targeted antibodies, which have confirmed TL1A’s role as a significant inflammatory pathway,” stated John Desjarlais, Ph.D., executive vice president and chief scientific officer at Xencor. “Our preclinical findings indicate that the in vitro potency of XmAb942 is on par with or exceeds that of first-generation anti-TL1A antibodies. Additionally, XmAb942 showcases enhanced pharmacokinetics, exhibiting a half-life of 23 days in non-human primates. We believe this supports a potential dosing schedule of eight to twelve weeks in humans, potentially enhancing convenience and adherence within the TL1A antibody landscape.”
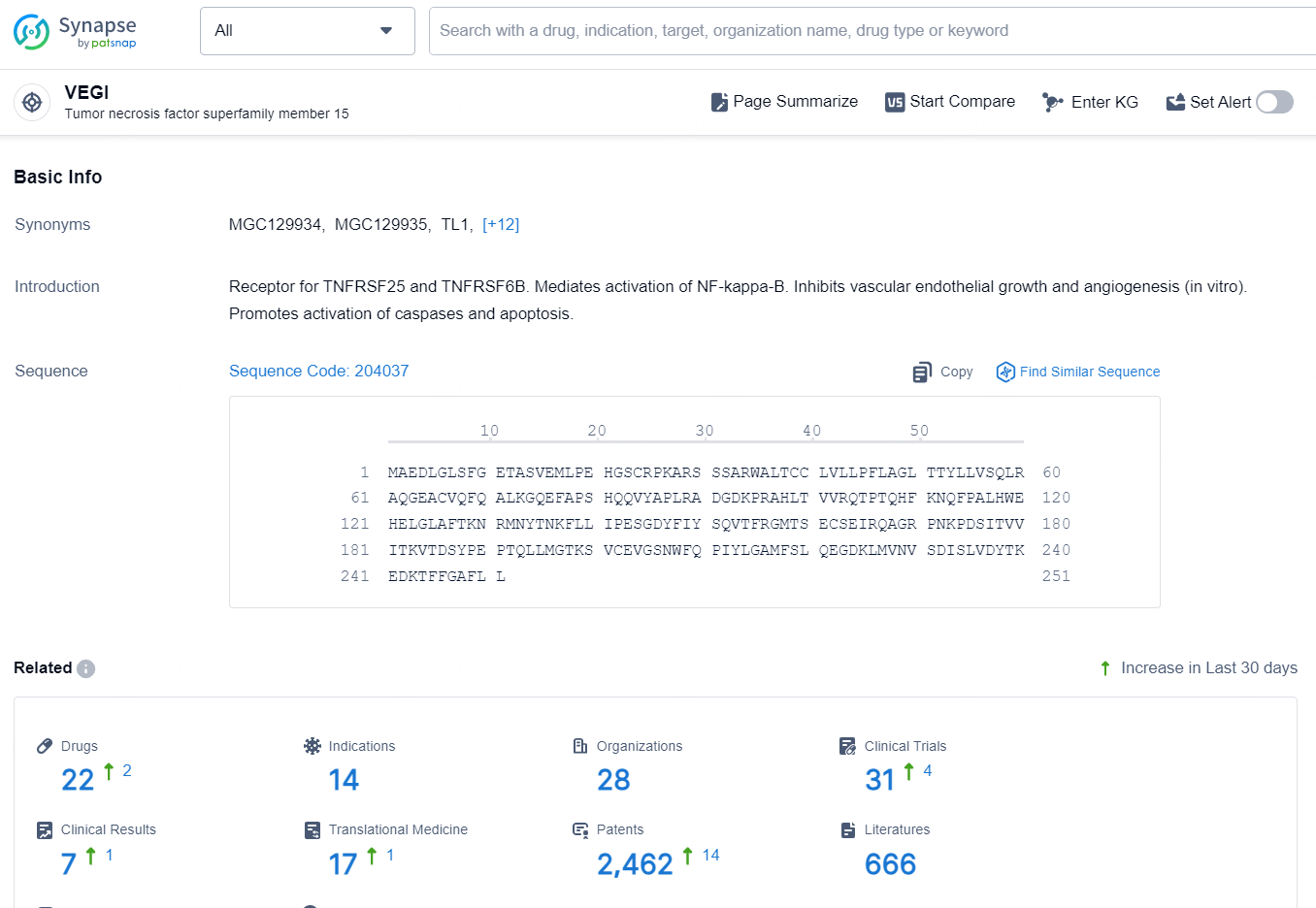
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 10, 2024, there are 22 investigational drugs for the VEGI targets, including 14 indications, 28 R&D institutions involved, with related clinical trials reaching 31, and as many as 2462 patents.
XmAb942(Xencor) is a monoclonal antibody drug developed by Xencor, Inc. It targets VEGI and has shown potential in the treatment of immune system diseases and digestive system disorders. The drug is specifically indicated for colitis, ulcerative, and inflammatory bowel diseases.