Foghorn Therapeutics Reveals Promising Cancer Treatment Advances with FHD-909 and Novel CBP/EP300 Degraders
Foghorn® Therapeutics Inc., an innovative biopharmaceutical firm in the clinical phase, focusing on the creation of novel therapeutic compounds that aim to ameliorate severe conditions through the rectification of dysfunctional gene expression patterns, disclosed novel preclinical findings regarding its unique treatments. Among these are FHD-909, a precision inhibitor targeting BRM (SMARCA2), and programs dedicated to the targeted degradation of both CBP and EP300. These groundbreaking updates are to be presented at the AACR (American Association for Cancer Research) Annual Meeting in 2024. Furthermore, the executive team of Foghorn will orchestrate a discussion via a conference call and simultaneously streamed webcast at 5 p.m. Eastern Time today to deliberate on significant advancements within the company's developmental portfolio.
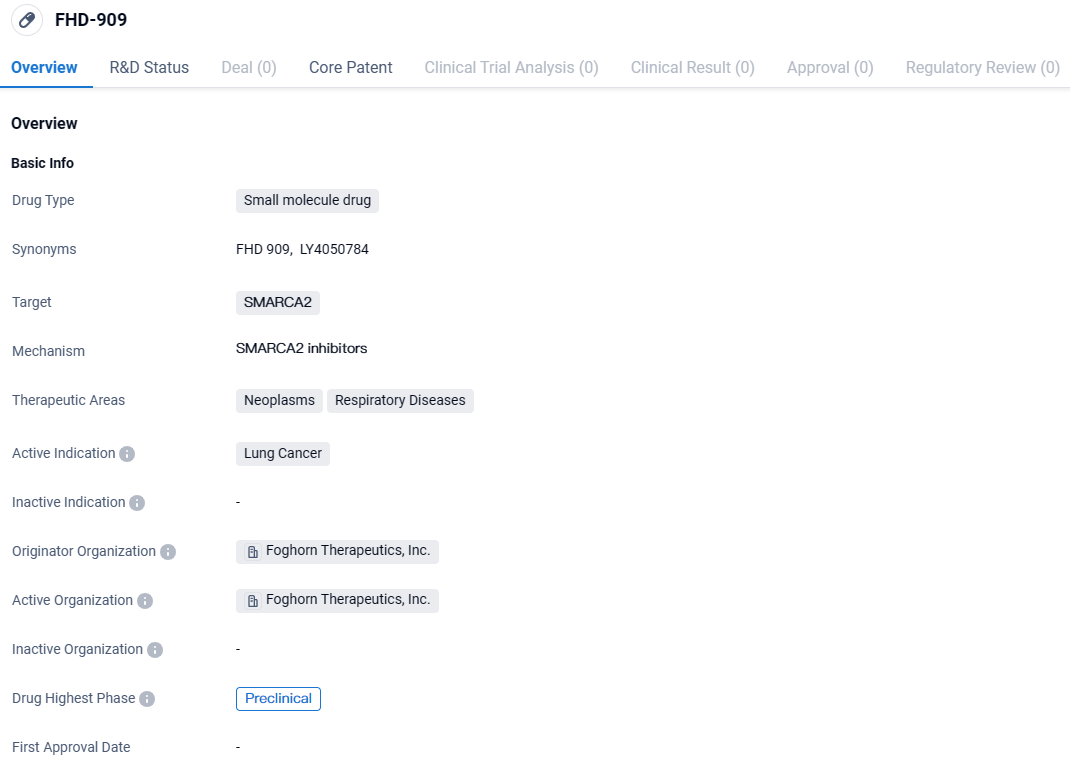
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Foghorn's Adrian Gottschalk, the esteemed President and CEO, expressed his satisfaction with the positive outcomes achieved from their precision-engineered therapeutic agents, specifically tailored to combat cancerous cells once deemed resilient to treatment. "The data regarding our cutting-edge BRM-targeting drug FHD-909, a trendsetter in its category, is particularly compelling," Gottschalk elucidated.
"This drug has displayed hopeful signs of mitigating effects and a proportional response based on dosage when assessed in preclinical experiments on cancer models with BRG1 anomalies. We're optimistic about its capabilities in pioneering novel cancer therapies, especially for patients with BRG1 mutant non-small cell lung cancer, a subgroup that comprises nearly 10% of such cases. With an Investigational New Drug (IND) application for FHD-909 on schedule for submission in the upcoming quarter, we anticipate advancing through phases with our ally Lilly."
Dr. Steve Bellon, the esteemed Chief Scientific Officer at Foghorn Therapeutics, commented on another promising venture: "Situated earlier in the pipeline, our EP300-targeting initiative is exhibiting optimistic signs of preclinical success. Unlike therapies with dual-targeting modalities that commonly prompt thrombocytopenia or impair megakaryocyte health, our approach does not trigger these adverse effects. Additionally, the longevity of our drugging agents is set to improve owing to our expertise in formulating long-acting drugs, a factor that may bolster the performance of our degrader portfolio when it comes to clinical applications."
BRM and BRG1, being essential yet non-interchangeable parts of the BAF complex, share a high degree of similarity, with mutations in BRG1 appearing across numerous tumor profiles, including in roughly 10% of non-small cell lung cancer cases. The reliance of these tumors on BRM activity for survival makes the selective disruption of BRM a tactful method for eliminating cancer cells, while sparing healthy tissue. Nevertheless, the challenge of distinguishing a selective BRM inhibitor is underscored by the fact that the ATPase domains within BRM and BRG1 hold an astonishing 92% sequence similarity.
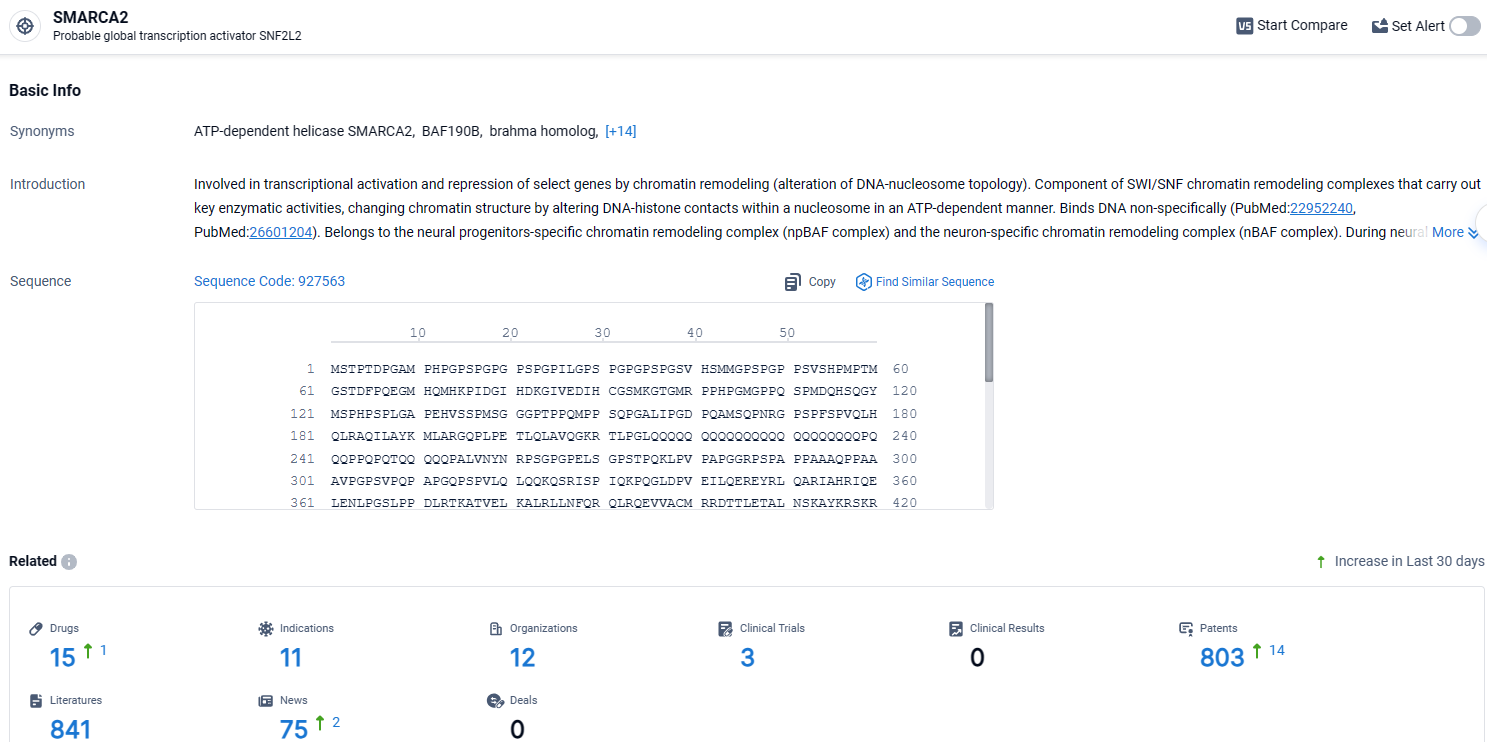
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of April 11, 2024, there are 15 investigational drugs for the SMARCA2 target, including 11 indications, 12 R&D institutions involved, with related clinical trials reaching 3, and as many as 803 patents.
FHD-909 targets SMARCA2 and is primarily intended for the treatment of lung cancer. Currently in the preclinical stage, FHD-909 shows promise as a potential therapeutic option for lung cancer patients. Its specific mechanism of action and potential application in respiratory diseases make it an interesting candidate for further investigation and development.