PeptiDream, a company specializing in the research and development of peptide drugs
PeptiDream is a biotechnology company based in Japan, focused on the development of innovative macrocyclic peptide drugs. Since its establishment in 2006, PeptiDream has been utilizing its proprietary Peptide Discovery Platform System (PDPS) technology, which efficiently generates highly diversified (trillions of varieties) non-standard peptide libraries designed to identify highly potent and highly selective macrocyclic peptide candidates. These candidates can subsequently be developed into therapeutic and diagnostic products based on peptides, small molecules, peptide-drug conjugates (PDCs), and multifunctional peptide conjugates (MPCs).
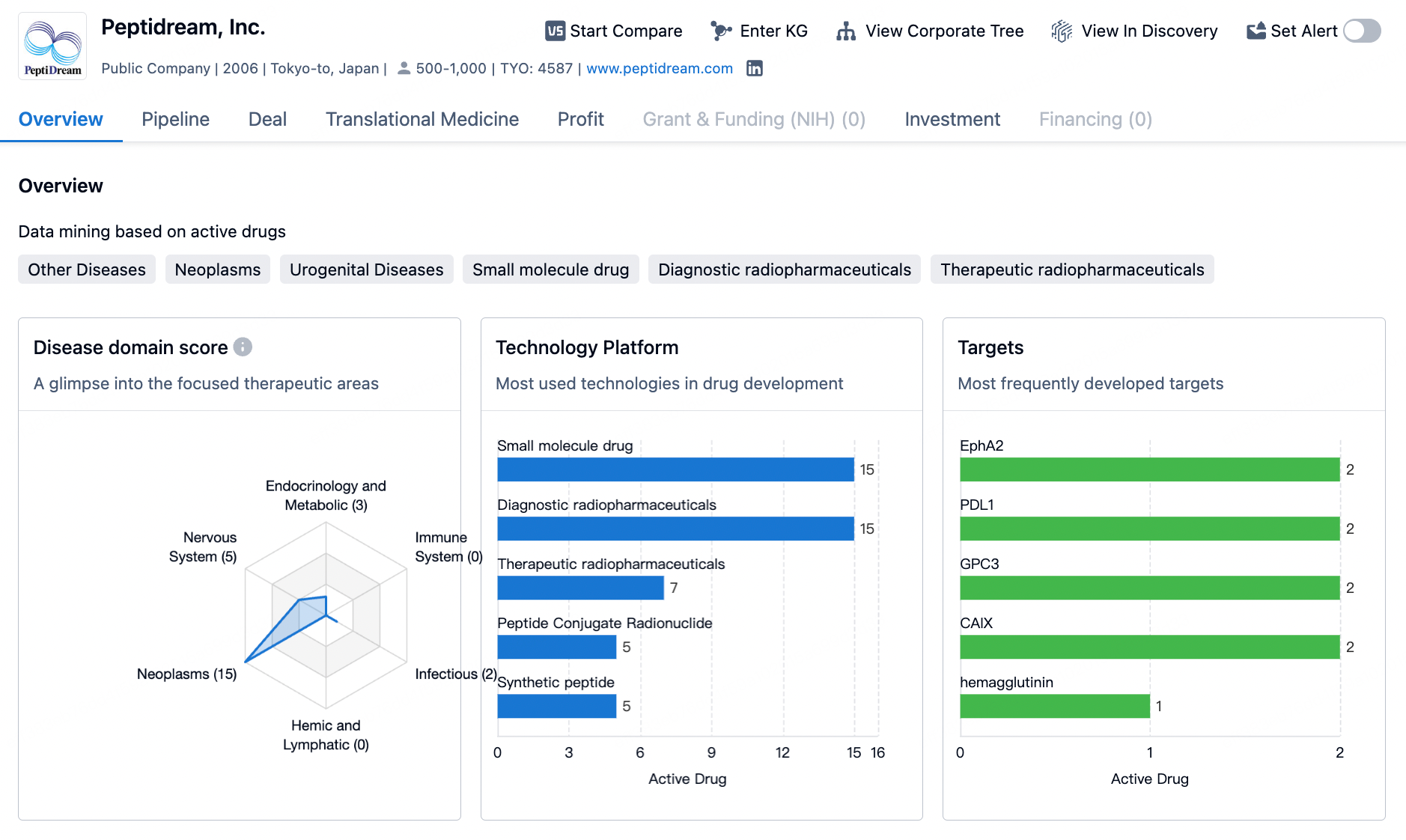
Leveraging its leading technology platform, PeptiDream has built an extensive global R&D partner network, facilitating the development and commercialization of a diverse and multi-faceted pipeline of investigational therapeutic drugs. Its commercial partners include multinational pharmaceutical companies such as Eli Lilly, Novartis, Merck, BMS, and many others.
The following reference material is from a company presentation by PeptiDream updated in February 2024:
Nuclear medicine-related business accounts for more than half of the company’s revenue.
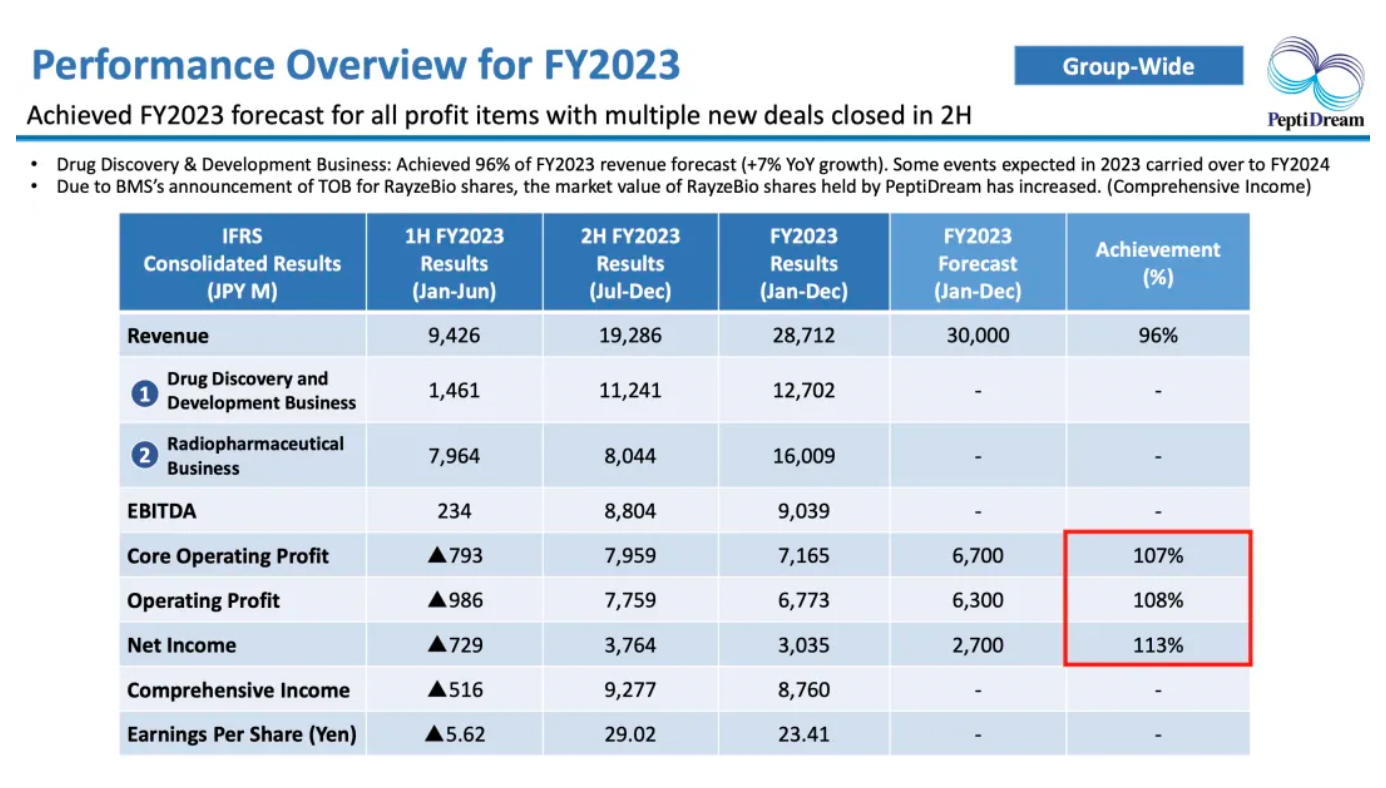
In 2023, the company collaborated with Genentech and Astellas on multi-target nucleic acid drugs, securing substantial initial payments. At the same time, the company also received milestone payments at the GLP-Tox stage from its collaboration with Novartis.
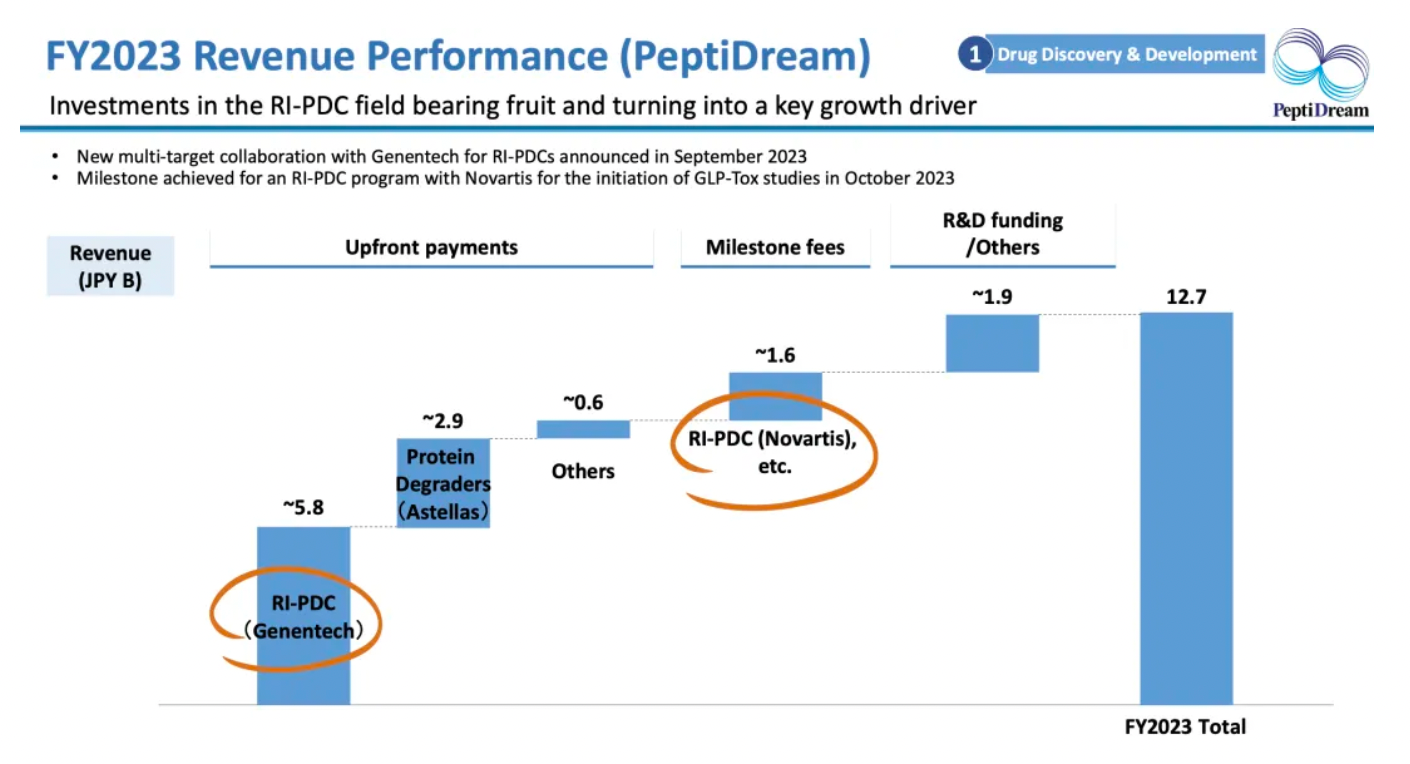
The company's wholly-owned subsidiary, PDRadiopharma, achieved year-over-year growth despite facing a challenging market.
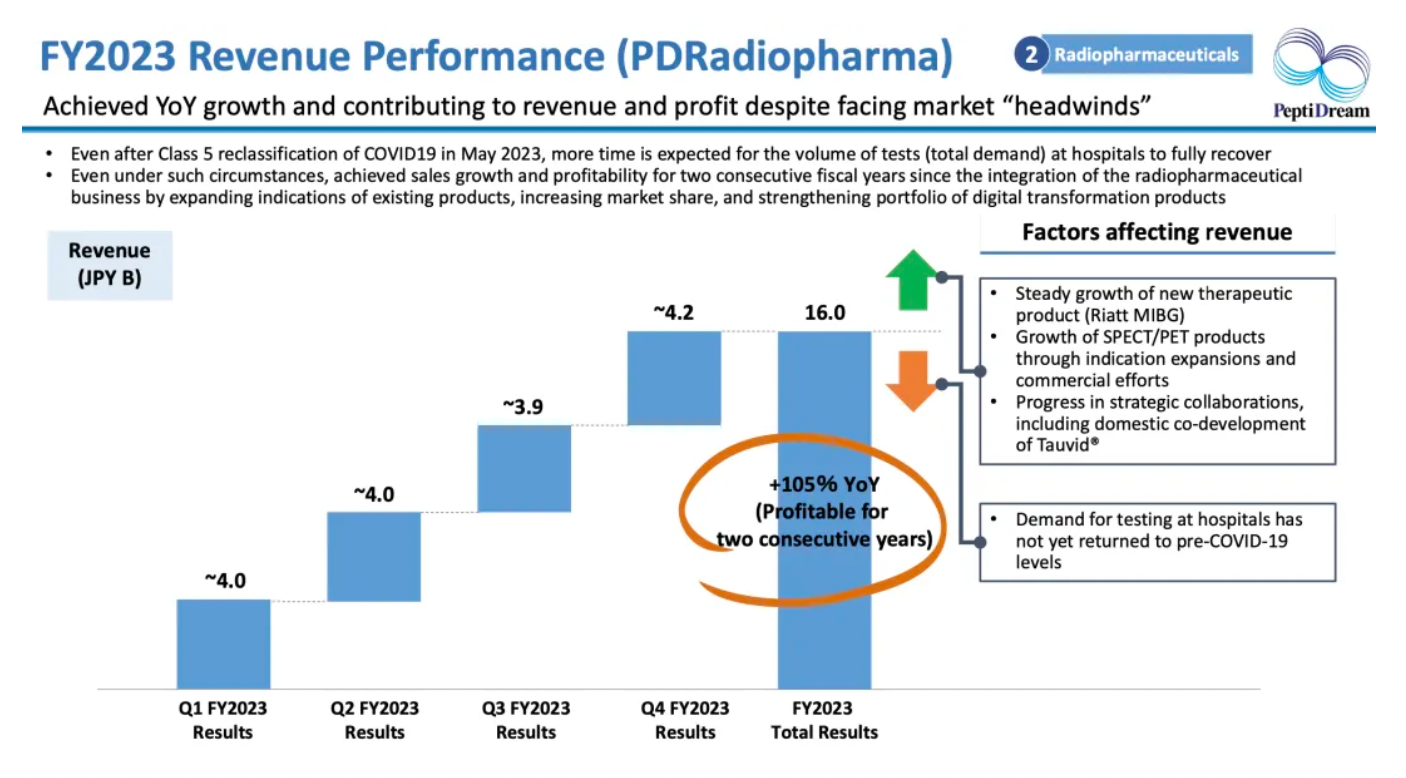
Key Advances in Nuclear Medicine: Major pharmaceutical companies are entering the field of nuclear medicine, pinning hopes on next-generation cancer treatment methods. The company has recently selected two projects as development candidates. The scope of AMYViD® has been expanded to include patients with mild cognitive impairment (MCI) or those suspected of having dementia due to Alzheimer's disease. Additionally, collaborations with Novartis, Genentech, and RayzeBio (BMS) have been expanded.

Pipeline Updates in Nuclear Medicine
64Cu-ATSM: This is a radiopharmaceutical conjugate consisting of lipophilic diacetyl-bis(N4-methylthiosemicarbazone) (ATSM) chelated with the positron and beta-emitting isotope Copper-64 (64Cu), selectively targeting hypoxic tissues with anticancer activity. Due to its high membrane permeability and redox potential, 64Cu-ATSM is preferentially taken up by hypoxic rather than normoxic cells. The radioactive copper component delivers selective cytotoxic beta radiation doses to hypoxic tumor cells, thereby effecting targeted cytotoxicity. 64Cu-ATSM serves not only as a hypoxia indicator for diagnostic purposes (assessing tumor hypoxia status through PET imaging) but also potentially offers therapeutic effects directly to hypoxic tumor cells, displaying dual functionality for theranostics (therapy and diagnostics combined).
177Lu/68Ga-Integrin (FF58): This therapy targets patients with advanced or metastatic tumors that express specific proteins (namely, integrins αvβ3 and αvβ5). [68Ga]Ga-FF58 primarily acts as a radioactive ligand imaging agent to identify tumor lesions.
18F-Flortaucipir (Tauvid): This is an FDA-approved radioactive diagnostic agent used for positron emission tomography (PET) imaging to visualize pathological Tau protein accumulations in the brain, particularly useful for patients being evaluated for Alzheimer’s disease.
18F-PD-L1 (BMS-986229): The compound 18F-BMS-986229, utilizing PET imaging technology, competitively binds with a macrocyclic peptide inhibitor targeting PD-L1 (BMS-986189) at various dosing levels, thus facilitating the assessment of the involvement of PD-L1 targets in tumors.
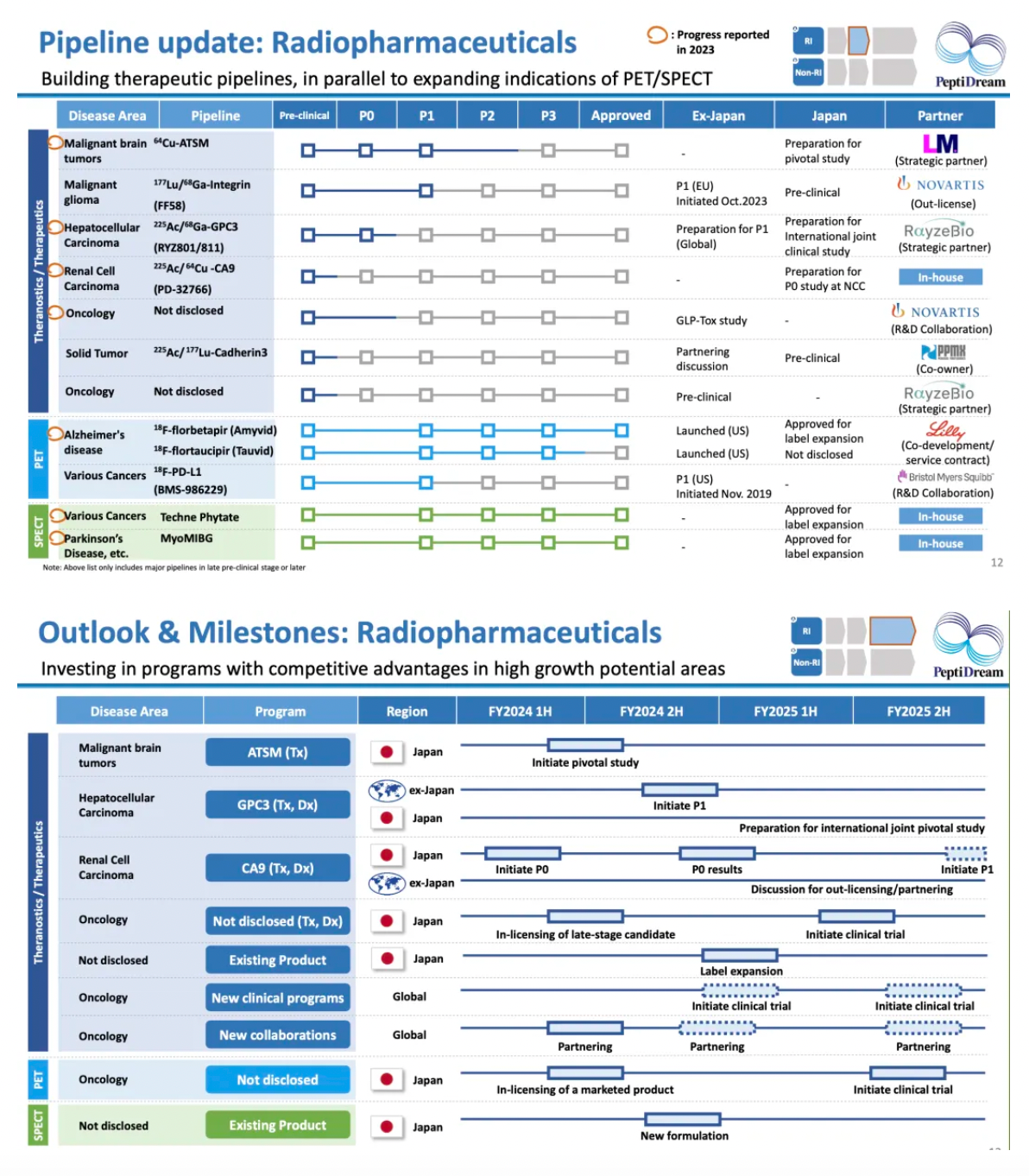
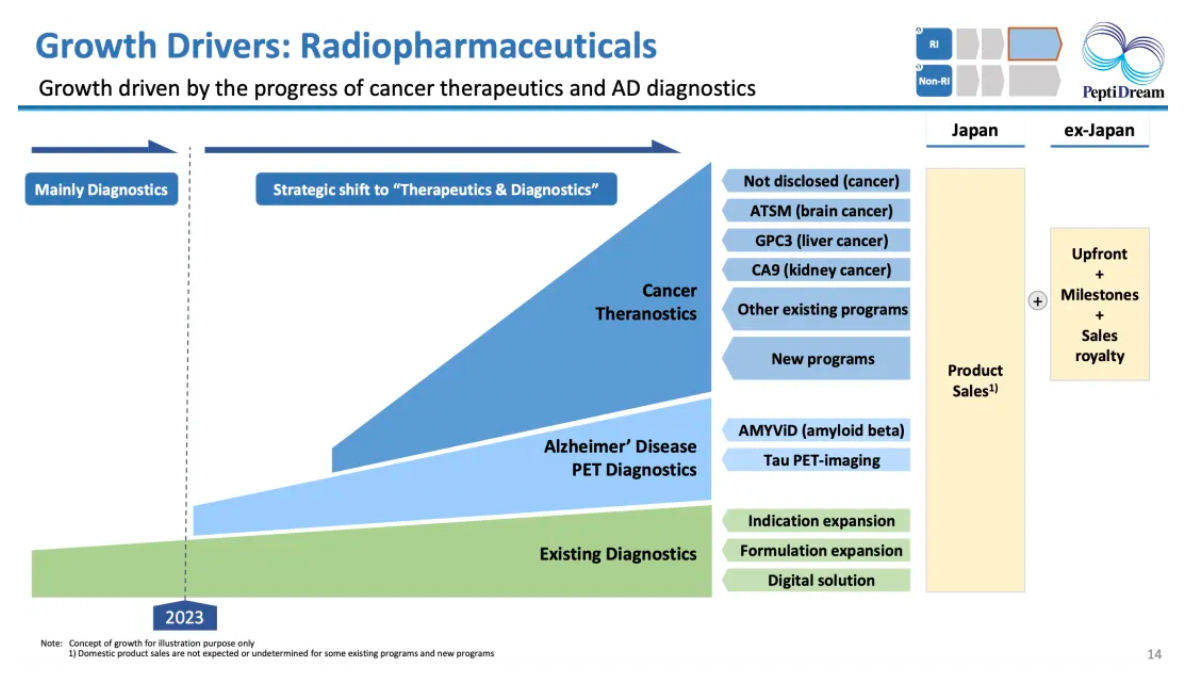
The year 2023 marks a new era in the global nuclear medicine field. PeptiDream has emerged as a leader due to its long-term focus on the development of cyclic peptides and radiodiagnostic tools, with therapeutic nuclear medicine becoming a new strategic pinnacle.
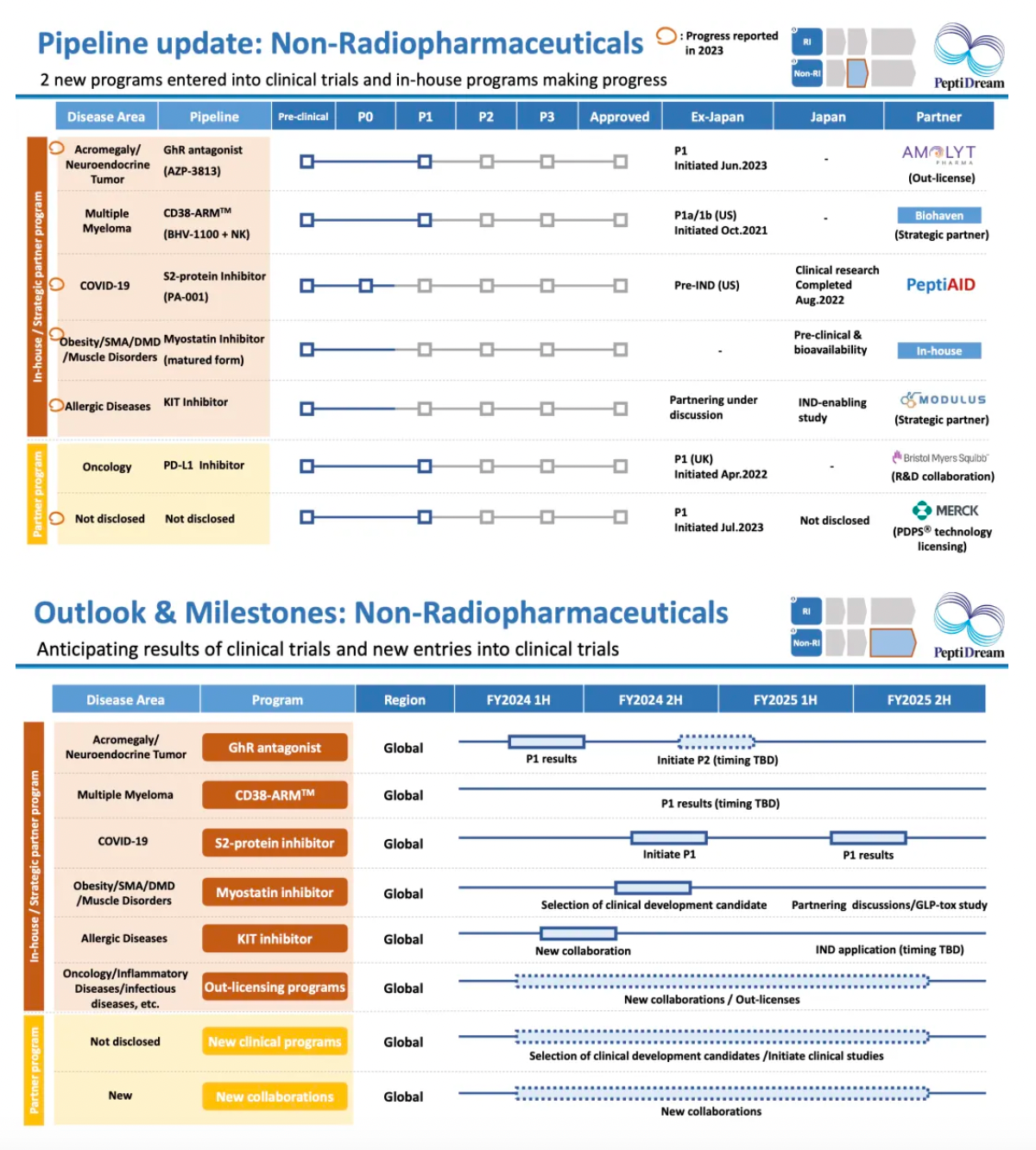
In the non-nuclear medicine direction, we are expanding ongoing collaborations with external pharmaceutical companies through our cyclic peptide-based drug development platform. The collaborators include MSD, Amolyt, Bayer, Janssen, Astellas, and others.

Updates to the pipeline in the non-nuclear medicine direction include key targets and developments such as GhR antagonists, Myostatin inhibitors, PD-L1 inhibitors, and KIT inhibitors.
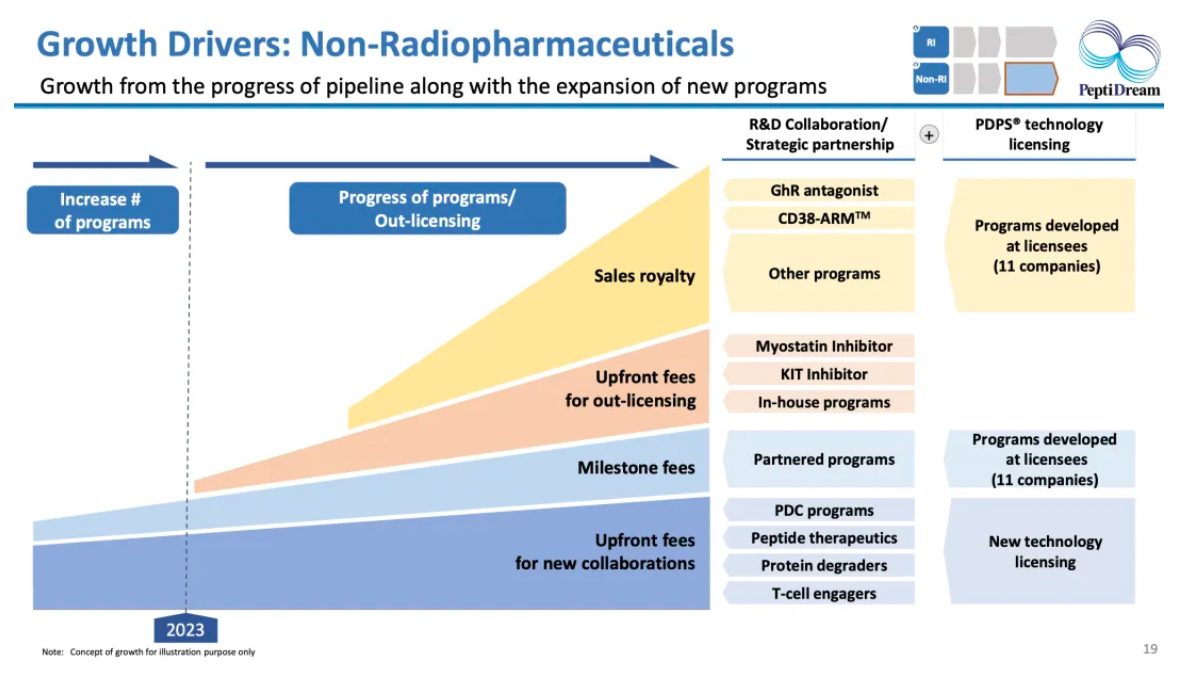
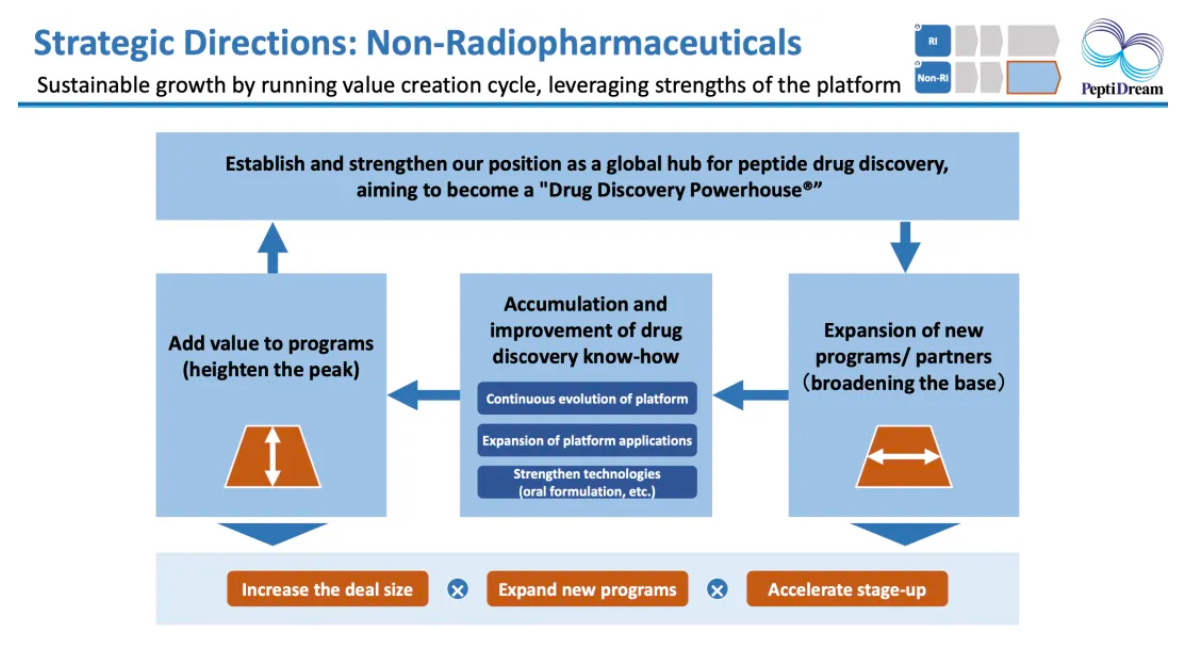
Surrounding the development platform of cyclic peptide drugs, achieve sustainable growth by leveraging its advantages.
For more information about PeptiDream, please click the image below to access the Synapse database.