Galapagos Reveals Promising CD19 CAR-T Therapy Results for Non-Hodgkin Lymphoma at EHA 2024
Galapagos NV revealed that they will share promising new results from the current Phase 1/2 ATALANTA-1 trial of their CD19 CAR-T therapy candidate, GLPG5101, for relapsed/refractory non-Hodgkin lymphoma at the European Hematology Association 2024 Hybrid Congress. The GLPG5101 candidate is generated through the company’s cutting-edge, decentralized T-cell production platform.
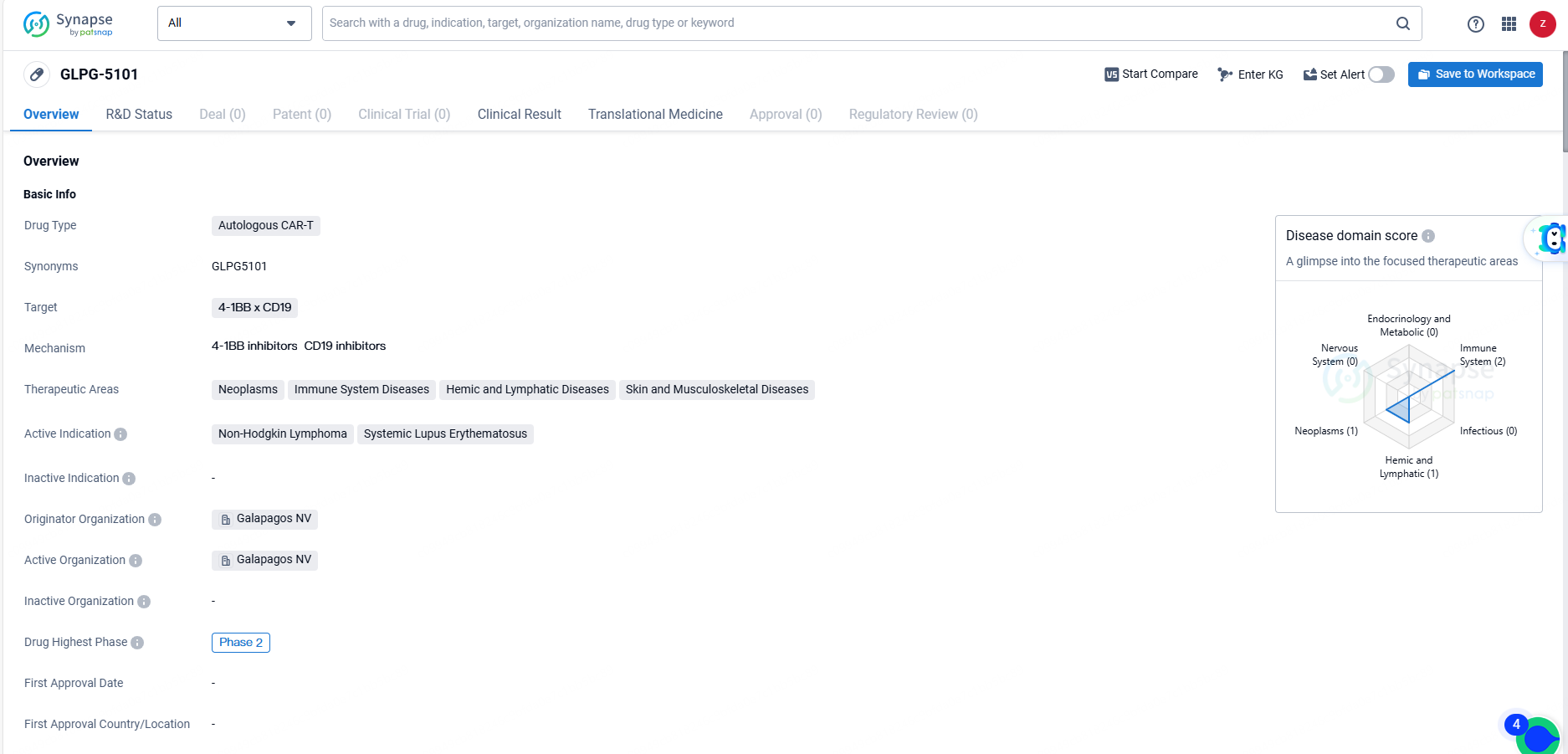
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The oral presentation contains the latest safety and efficacy data for GLPG5101 in patients with diffuse large B-cell lymphoma, follicular lymphoma, marginal zone lymphoma, and mantle cell lymphoma. It also features information on durability and cellular kinetics. As of the data cut-off on December 20, 2023, no unexpected safety issues were noted, and GLPG5101 achieved high complete response rates across all conditions in this heavily pretreated patient group.
In 94% of cases, GLPG5101 was administered as a fresh product with a median vein-to-vein time of seven days, thus eliminating the requirement for bridging therapy. T-cell subsets were evaluated in both the apheresis starting material and the final CAR-T product. The final product showed a higher proportion of early phenotypes of CD4+ and CD8+ CAR T cells compared to the starting material, indicating an increase in these populations during manufacturing. This highlights the effectiveness of Galapagos' decentralized manufacturing platform in delivering a high-quality CAR T-cell product to patients.
"We are thrilled to present promising new data for GLPG5101 at the EHA congress. The high complete response rates, coupled with low-grade CRS and ICANS, underscore the potential of GLPG5101 in meeting the critical needs of this patient population," said Dr. Jeevan Shetty, M.D., Head of Clinical Development Oncology at Galapagos.
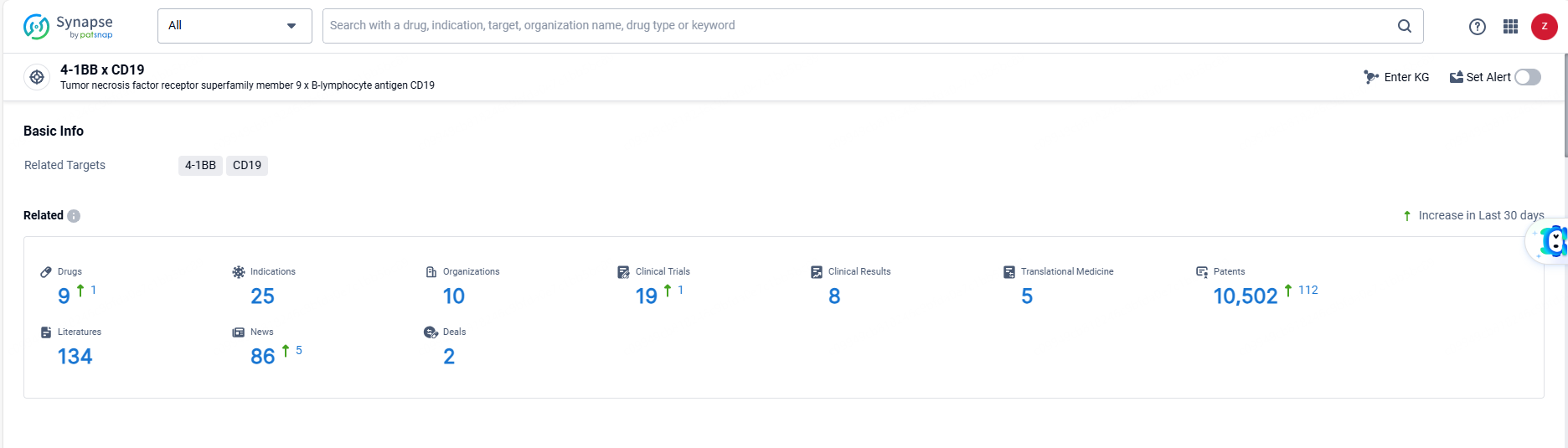
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 19, 2024, there are 9 investigational drugs for the 4-1BB and CD19 target, including 25 indications, 10 R&D institutions involved, with related clinical trials reaching 19, and as many as 10502 patents.
GLPG-5101 represents a promising candidate in the field of biomedicine, with its innovative approach to utilizing CAR-T therapy for the treatment of Non-Hodgkin Lymphoma and Systemic Lupus Erythematosus. The drug's specific targeting of 4-1BB x CD19 and its potential impact on a range of therapeutic areas underscore its significance in addressing unmet medical needs in oncology and immunology.