Galderma's Nemolizumab for Prurigo Nodularis and Eczema Approved for Submission in US and EU
Galderma has made a recent announcement regarding the acceptance of its Biologics License Applications by the U.S. Food and Drug Administration. These applications pertain to the use of nemolizumab, which is a proposed treatment for individuals suffering from prurigo nodularis, as well as adolescents and adults diagnosed with atopic dermatitis ranging from moderate to severe intensity.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
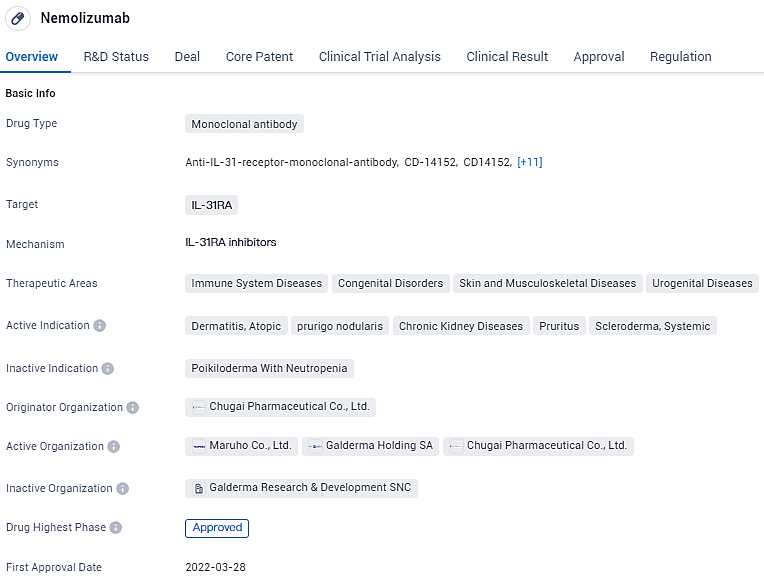
"Nemolizumab is an innovative investigational monoclonal antibody crafted to target and impede IL-31 signaling pathways, aiming to provide effective and swift alleviation of the most troublesome symptom faced by patients with certain dermatological conditions: itching.
The Food and Drug Administration (FDA) in the United States has recognized the potential of nemolizumab in addressing prurigo nodularis by awarding it with a Priority Review status. This accolade builds upon its earlier Breakthrough Therapy designation for addressing itch related to prurigo nodularis, an acknowledgment first given in December 2019 and reaffirmed in March 2023. Drugs that receive Priority Review are those anticipated to offer significant enhancements in the treatment, diagnosis, or prevention of severe illnesses.
The European Medicines Agency likewise has acknowledged the significance of this treatment by accepting Marketing Authorization Applications from Galderma for using nemolizumab in the management of prurigo nodularis as well as atopic dermatitis. Looking ahead, Galderma is gearing up for several regulatory filings slated for the year 2024.
"The constant itching that plagues individuals with prurigo nodularis and atopic dermatitis can take a heavy toll on their life's quality. Our gratitude extends to the patients and health professionals who have contributed with valuable perspective fuelling our clinical research. These studies have been pivotal in evaluating the efficacy of nemolizumab in mitigating itch and skin eruptions. As we approach the possibility of introducing this trailblazing therapy to those who stand to benefit from it, we eagerly anticipate the decisions regarding these submissions."
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
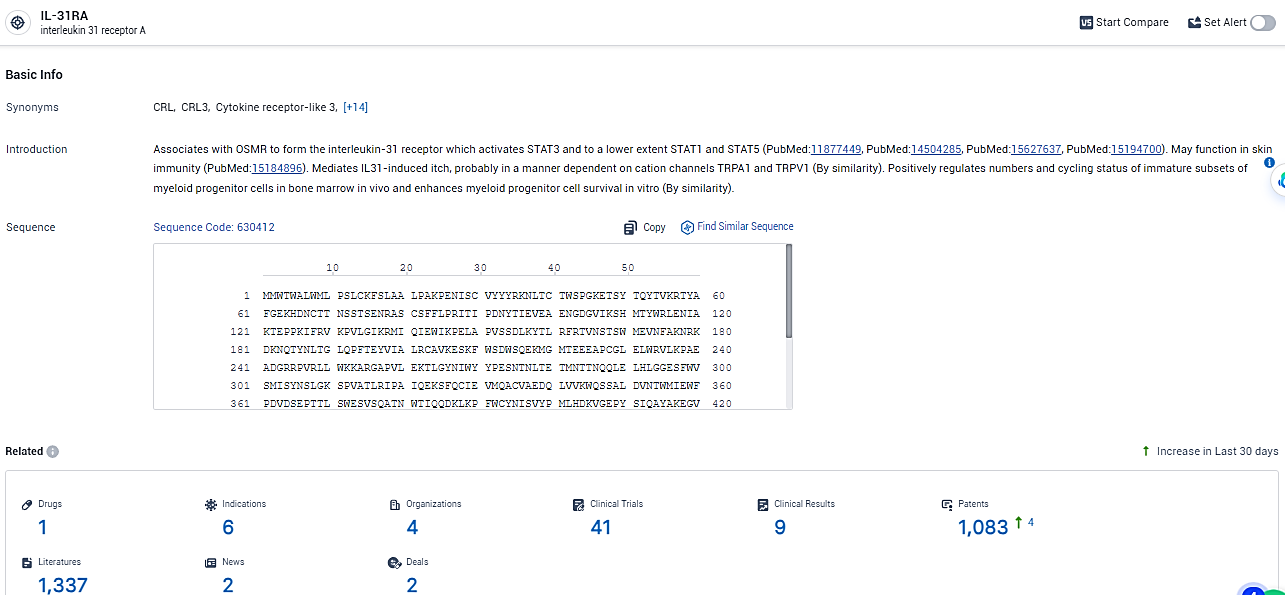
According to the data provided by the Synapse Database, As of February 19, 2024, there are 1 investigational drugs for the IL-31RA target, including 6 indications, 4 R&D institutions involved, with related clinical trials reaching 41, and as many as 1083 patents.
Nemolizumab has been approved for various therapeutic areas, including immune system diseases, congenital disorders, skin and musculoskeletal diseases, and urogenital diseases. It has shown efficacy in treating dermatitis, atopic prurigo nodularis, chronic kidney diseases, pruritus, and scleroderma systemic. Nemolizumab received its first approval in Japan in 2022 and has been designated as a breakthrough therapy, indicating its potential to address unmet medicine.