Genentech's Alecensa has achieved remarkable Phase III outcomes for individuals diagnosed with early-stage lung cancer that is positive for the ALK mutation
Genentech, a subsidiary of the Roche Group, announced today that the Phase III ALINA study assessing Alecensa® (alectinib) has achieved its primary endpoint of disease-free survival (DFS) during a pre-determined interim analysis. As adjuvant therapy for individuals with completely resected Stage IB (tumor ≥ 4 cm) to IIIA anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer (NSCLC), Alecensa has displayed a statistically significant and clinically meaningful enhancement in DFS. In a Phase III trial, Alecensa is the first and sole ALK inhibitor to exhibit a decrease in the risk of disease recurrence or mortality for those with early-stage ALK-positive NSCLC.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The overall survival (OS) data were still in their early stages of development during the time of this analysis. No unforeseen safety issues were observed. The findings from the ALINA study will be presented at an upcoming medical conference and submitted to regulatory authorities worldwide, including the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
Levi Garraway, M.D., Ph.D., the chief medical officer and head of Global Product Development, stated, "Alecensa has already revolutionized outcomes for individuals with advanced ALK-positive NSCLC, and these impressive results provide evidence for the first time that this medication could also have a critical role in early-stage disease, where there remains a significant unmet need. If approved, Alecensa has the potential to target cancer before its metastasis, allowing for improved chances of cure. This aligns with our ultimate goal at Genentech, and we eagerly anticipate sharing these findings with regulatory agencies to expedite availability to patients."
Currently, despite adjuvant chemotherapy, approximately 45-76% of individuals with early-stage lung cancer experience cancer recurrence after surgery. Recent advancements in treatment, such as immunotherapies, have shown promising results for some patients with early-stage NSCLC. However, there are currently no approved ALK inhibitors specifically for early-stage ALK-positive disease. ALK-positive NSCLC is most commonly found in younger individuals, typically aged 55 or below, with a limited smoking history or who are non-smokers. The National Comprehensive Cancer Network® (NCCN®) Clinical Practice Guidelines in Oncology (NCCN Guidelines®) recommend biomarker testing for ALK rearrangements in resected surgical tissue or biopsy samples from patients with Stage IB to IIIA and IIIB NSCLC, both in the advanced and early-stage settings.
About the ALINA study
The ALINA study [NCT03456076] is a Phase III clinical trial that aims to assess the effectiveness and safety of adjuvant Alecensa® (alectinib) in comparison to platinum-based chemotherapy for individuals with completely resected Stage IB (tumor ≥ 4 cm) to IIIA (UICC/AJCC 7th edition) anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer (NSCLC). This study is a randomized, active-controlled, multicenter, and open-label study, involving a total of 257 patients who were randomly assigned to either the investigational or control treatment group. The primary goal of this study is to determine the disease-free survival (DFS), while secondary outcome measures include overall survival (OS) and the percentage of patients experiencing adverse events.
About Alecensa®(alectinib)
Alecensa is indicated for the treatment of individuals diagnosed with metastatic non-small cell lung cancer (mNSCLC) harboring an abnormal anaplastic lymphoma kinase (ALK) gene. Prior to prescribing Alecensa, healthcare professionals will conduct appropriate testing to ensure its suitability for the patient.The safety and efficacy of Alecensa in pediatric patients have not been
About Genentech in lung cancer
Lung cancer remains a significant area of emphasis and financial investment for Genentech. Our dedication involves the advancement of novel strategies, medications, and diagnostic tests to offer valuable support to individuals affected by this life-threatening condition. Our primary objective revolves around providing efficacious treatment options for all individuals diagnosed with lung cancer. At present, we have a portfolio of six approved medications designed specifically to address certain subtypes of lung cancer. Additionally, we are actively engaged in the development of over 10 therapeutic agents targeting prevalent genetic drivers of lung cancer or aimed at enhancing the immune system's capacity to combat the disease. Genentech is resolute in its commitment to enhancing the treatment landscape for early-stage lung cancers, with the ultimate goal of improving curative prospects for a wider population.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
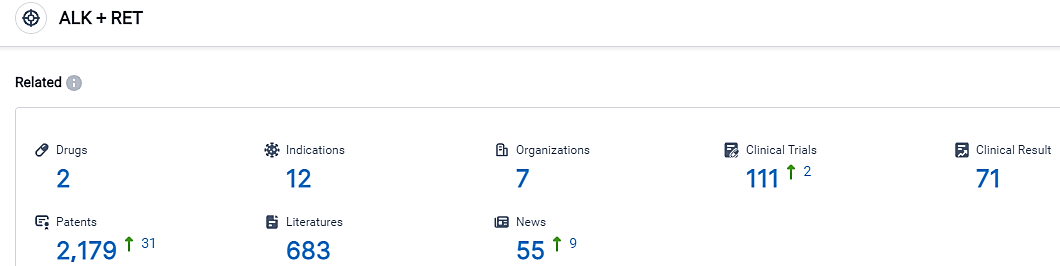
According to the data provided by the Synapse Database, As of September 2, 2023, there are 2 investigational drugs for the ALK and RET target, including 12 applicable indications, 7 R&D institutions involved, with related clinical trials reaching 111, and as many as 2179 patents. According to projections from the American Cancer Society, it is anticipated that over 238,000 individuals residing in the United States will receive a lung cancer diagnosis in the year 2023.
Non-small cell lung cancer (NSCLC) constitutes approximately 80-85% of all lung cancer cases. Prompt intervention at an early stage of the disease, prior to metastasis, holds the potential to impede disease recurrence and offer the most promising prospects for achieving a cure.