The U.S. has granted IND approval to HBM9033, the initial ADC project for solid tumors by Harbour BioMed
Harbour BioMed declared that the U.S. FDA has given the go-ahead for the IND application, permitting the initiation of clinical studies for its inaugural Antibody Drug Conjugate (ADC) project, HBM9033.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
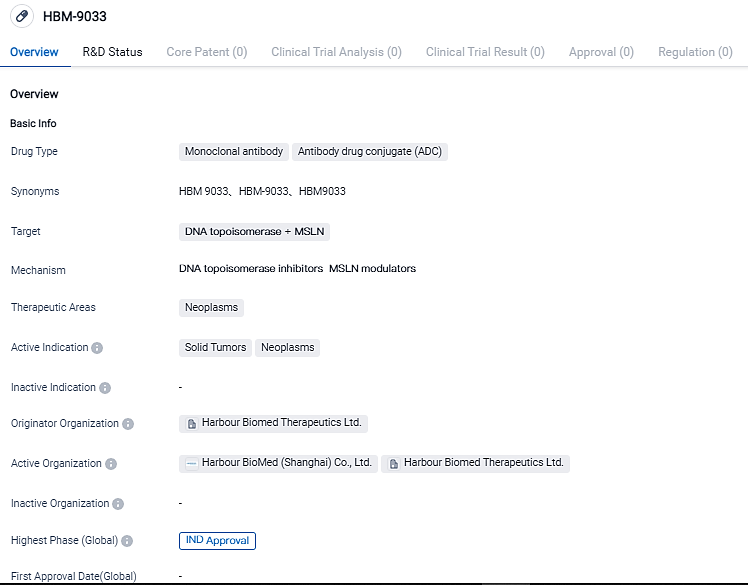
HBM9033 is an ADC drug contender designed to interact specifically with human mesothelin (MSLN), a tumor-associated antigen that is overexpressed in a variety of solid malignancies, including mesothelioma, ovarian cancer, lung cancer, breast cancer and pancreatic cancers.
The Harbour Mice® platform is used to produce the fully human monoclonal antibody in HBM9033. This antibody reacts preferentially with membrane-bound MSLN rather than soluble MSLN, reducing the obstruction caused by shedding MSLN on the attachment and absorption of the membrane-bound MSLN.
HBM9033 employs a cancer-specific breakable linker combined with a novel topoisomerase inhibitor for enhanced stability and effectiveness. HBM9033 has evidenced exceptional potency and safety in preliminary studies due to its unparalleled design for both the mAb and linker-payload. In conjunction with MediLink Therapeutics, the company developed this product.
The purpose of this Phase I study is to investigate HBM9033's safety, tolerability, pharmacokinetics, and anti-cancer properties in individuals with advanced solid tumors.Dr. Jingsong Wang, Founder, Chairman, and CEO of Harbour BioMed stated, "HBM9033 is our pioneering ADC product at the clinical trial stage and it holds promise for being the top MSLN targeting treatment". He further added, "Leveraging the comprehensive applications of the HCAb PLUSTM platform in the ADC realm, we have created an ADC ecosystem that integrates our in-house development with external alliances. Our swift advancement, as well as that of our partners, testifies to our enduring commitment to fortifying our standing in the global ADC field."
Harbour BioMed constitutes a worldwide corporation focusing on biopharmaceuticals, taking a dedicated role in discovering, manufacturing, and retailing innovative antibody treatments with an emphasis on immunology and oncology. The company enhances its abundant assemblage and unique procession through its in-house R&D skills, working in cooperation with allies for joint discovery and development, and occasionally pursuing strategic acquisitions.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
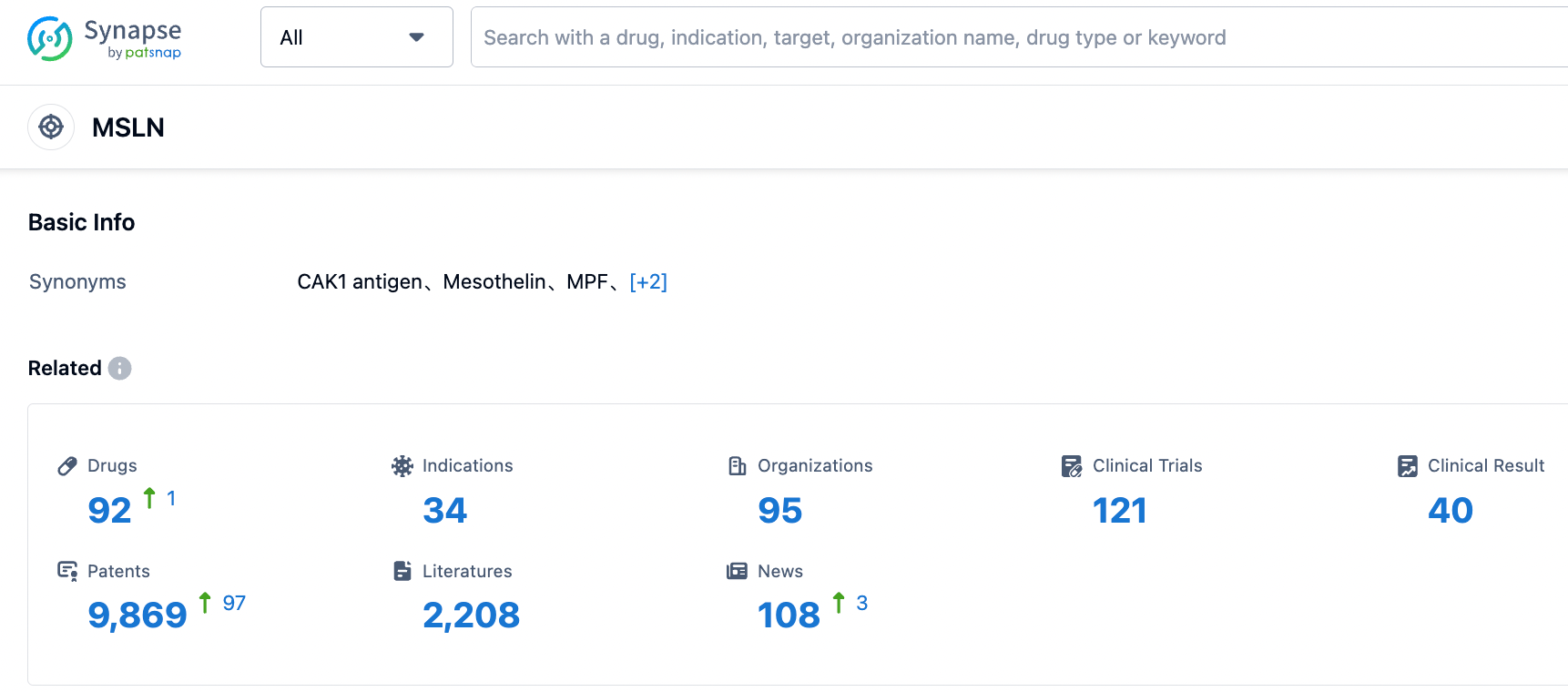
According to the data provided by the Synapse Database, As of September 4, 2023, there are 92 investigational drugs for the MSLN target, including 34 applicable indications, 95 R&D institutions involved, with related clinical trials reaching 121, and as many as 9869 patents.
Mesothelin (MSLN) is a tumor-associated antigen that is highly expressed in various malignant tumors such as pancreatic cancer, mesothelioma, lung cancer and ovarian cancer. It is involved in tumor proliferation, invasion and metastasis, and is widely recognized as a potential target for anticancer drug development.
Currently, some progress has been made in the development of drugs targeting MSLN. For example, MORAb-009 (amatuximab), a monoclonal antibody against MSLN, has shown therapeutic effects on some solid tumors in clinical trials. In addition, immunotherapies targeting MSLN such as CAR-T cell therapy and vaccines are also undergoing clinical trials.In the future, with the advancement of scientific research technology, we have reason to believe that novel therapies targeting MSLN will be further developed. For example, drug delivery systems combining immunotherapy, accurately targeting MSLN for drug delivery, may achieve better treatment effects.