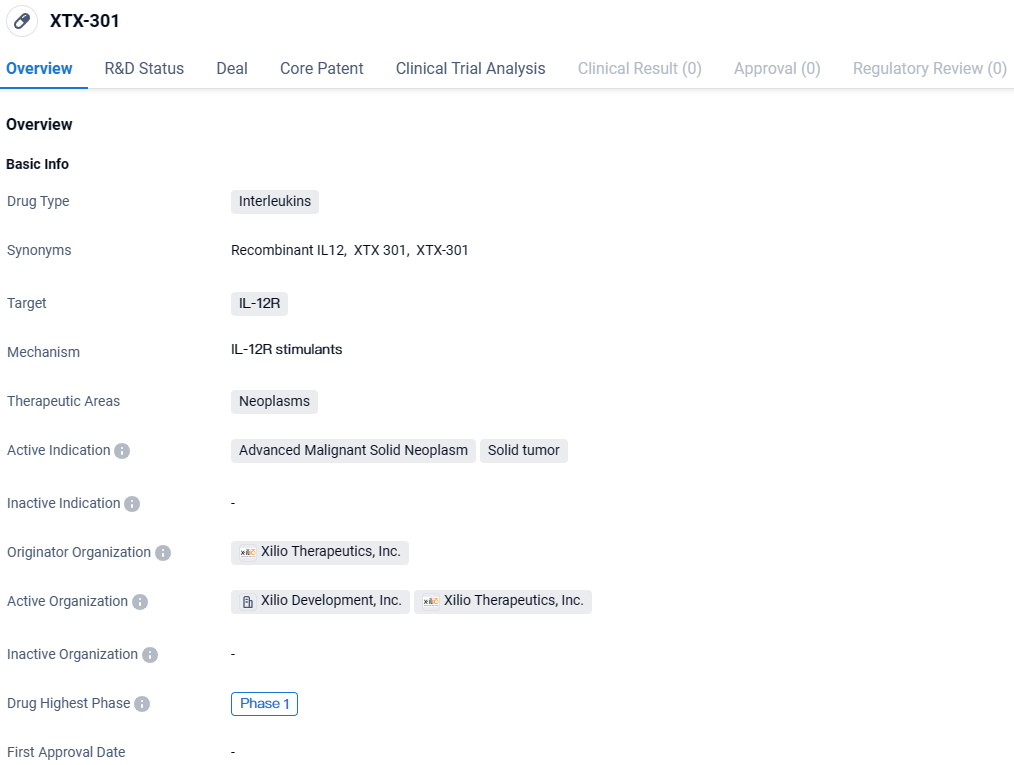
Gilead-Xilio Ink Deal on IL-12 Oncology Project
Gilead Sciences, Inc. has entered into a definitive agreement with Xilio Therapeutics, Inc. for the exclusive rights to advance and market Xilio’s investigational cancer therapy, known as XTX301, which is currently in Phase 1 clinical trials and utilizes a tumor-activated IL-12 mechanism.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Xilio Therapeutics, an enterprise at the clinical-trial phase in the biotech sector, is committed to the innovation and development of therapies for immuno-oncology that are activated by tumors. Utilizing a unique, proprietary platform that initiates tumor activation, Xilio is engineering a suite of groundbreaking, tumor-specific agents such as antibodies, cytokines, bispecific molecules, and cell engaging compounds. These agents are crafted to refine the therapeutic index and concentrate therapeutic actions within the tumor's localized environment.
"Xilio's cutting-edge technology for tumor activation is a strategic fit with Gilead's efforts in advancing treatments for challenging cancer types and intensifies our immersion in the realm of immuno-oncology," expressed Bill Grossman, MD, PhD, Senior Vice President at Gilead Sciences, overseeing Oncology Clinical Development. "There's a significant promise seen in IL-12 for addressing various forms of cancer, and we're poised to collaborate with Xilio on the development of XTX301, which is a novel tumor-specific IL-12, both as an independent and a combinatorial therapy for multiple solid tumor classes."
"Partnering with Gilead, a company that is confident in our tumor-targeting approach and their comprehensive clinical and commercial experience in pioneering immuno-oncology treatments, will undoubtedly propel the progress and reach of XTX301, our tumor-specific IL-12 variant," stated René Russo, Pharm.D., President and CEO of Xilio. "Working jointly with Gilead, we are eager to explore XTX301's capability to offer substantial relief to a spectrum of cancer types, particularly those that are immunologically unresponsive ‘cold’ tumors, and to subvert the traditionally severe side effects associated with IL-12."
XTX301 is an investigative, tumor-specific IL-12 therapeutic, crafted to robustly incite anti-tumor immune responses and transform the tumor microenvironment, particularly inducing a shift in immunologically inert "cold" tumors to a more reactive or "hot" state. Currently, Xilio is in the process of determining the safety and feasibility of XTX301 as a singular treatment in patients with advanced iterations of solid tumors. This investigational phase is part of a first-for-human, multi-site, non-restricted Phase 1 clinical evaluation.
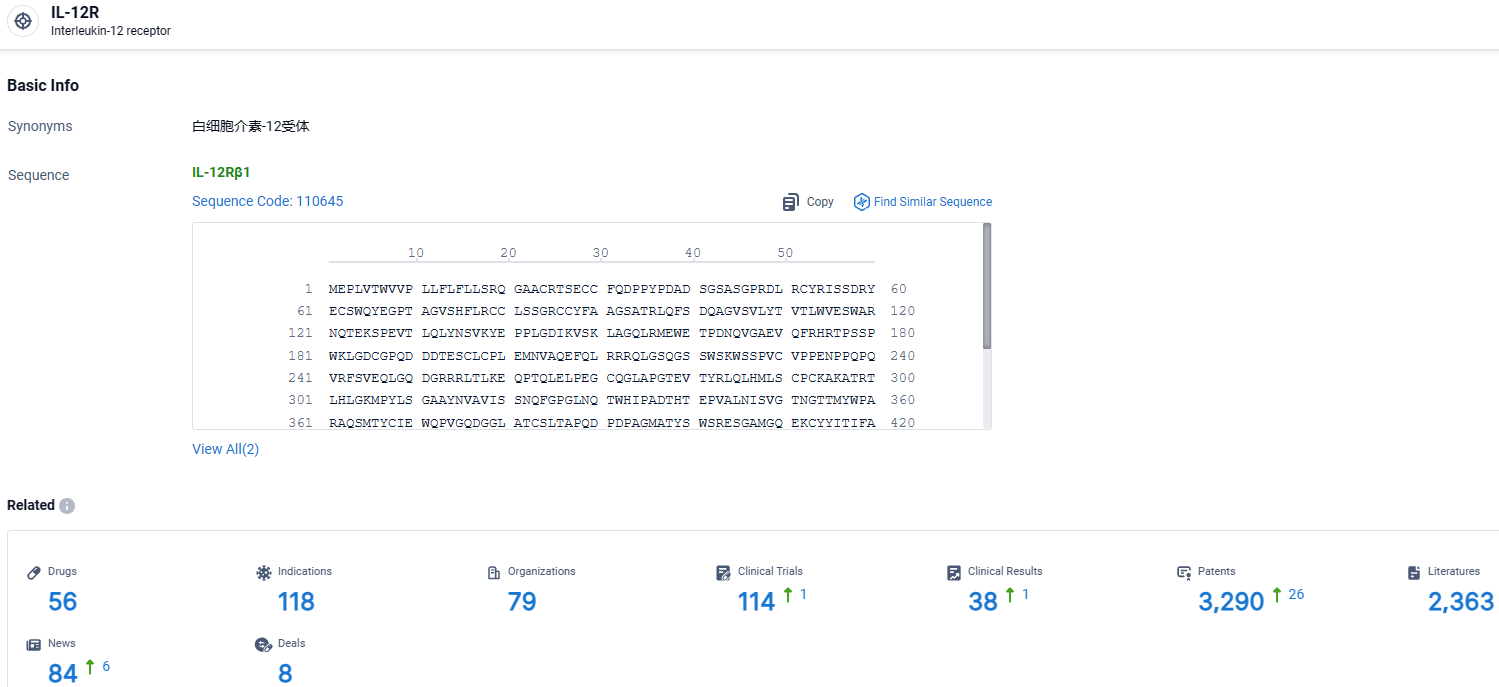
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of April 1, 2024, there are 56 investigational drugs for the IL12R target, including 118 indications, 79 R&D institutions involved, with related clinical trials reaching 114, and as many as 3290 patents.
XTX-301 is an Interleukin drug that targets the IL-12R and is being developed for the treatment of advanced malignant solid neoplasms and solid tumors. Currently in Phase 1 of development, XTX-301 shows potential as a new therapeutic option for patients with these types of cancers.