Givastomig: brief review of its R&D progress and the clinical result in 2023 ESMO
The recent ESMO Congress presented noteworthy clinical trial results of Givastomig, paving the way for a detailed exploration of its therapeutic potential in solid tumors.
Givastomig's R&D Progress
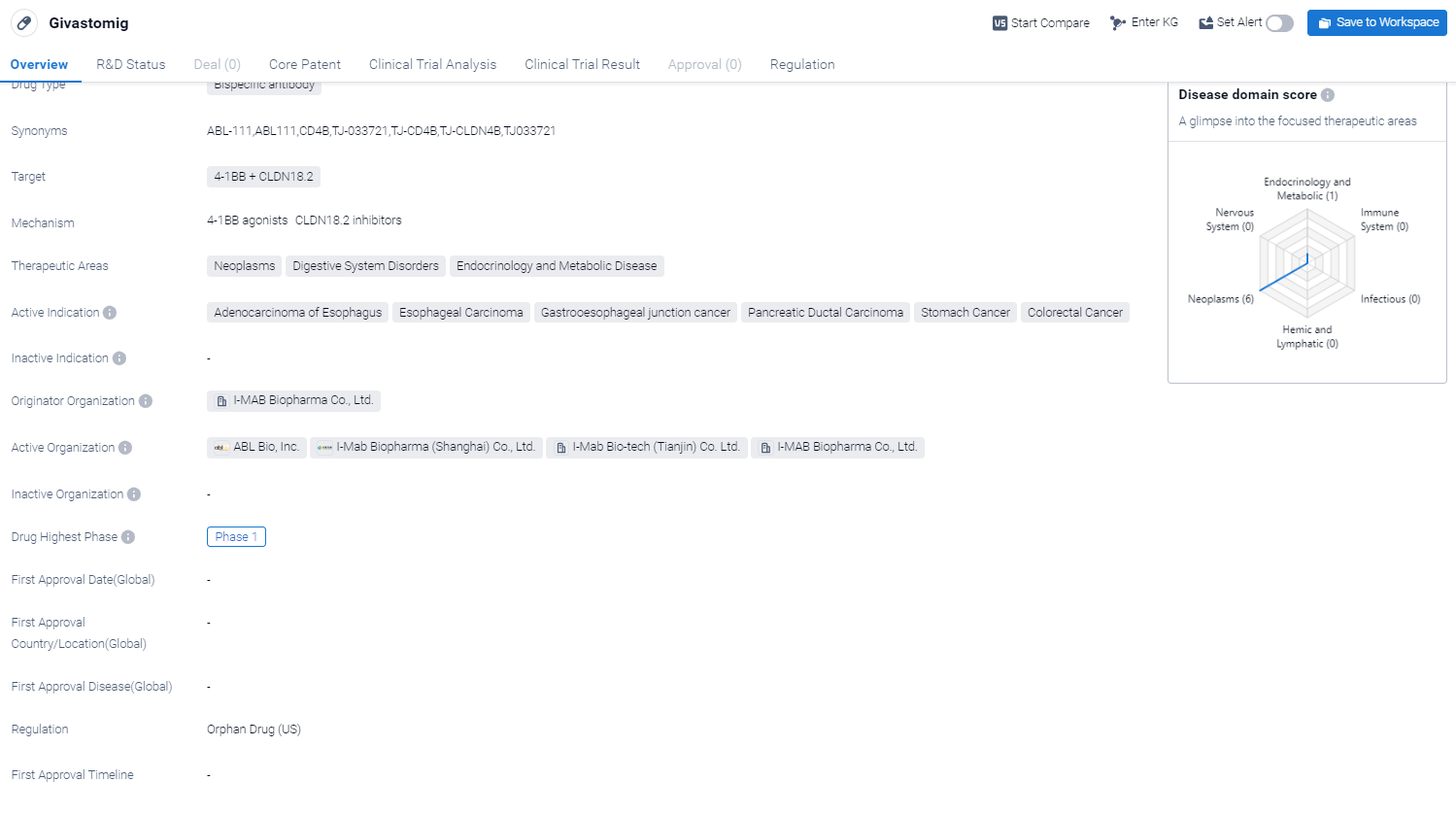
Givastomig is a bispecific antibody drug that targets two proteins, 4-1BB and CLDN18.2, in the field of biomedicine. It is being developed by I-MAB Biopharma Co., Ltd. The drug has shown potential therapeutic applications in various areas, including neoplasms, digestive system disorders, and endocrinology and metabolic diseases.
According to the Patsnap Synapse, Givastomig is currently in Phase 1 of clinical trials globally . And the clinical trial areas for Givastomig are primarily in the China and United States. The key indication is Metastatic Solid Tumor.
Detailed Clinical Result of Givastomig
The sequential assignment, open-labeled clinical trial (NCT04900818) was aimed to evaluate the safety, clinical response (RECIST v1.1), pharmacokinetics (PK), soluble 4-1BB (s4-1BB) levels, serum cytokines and immunophenotypes of Givastomig.
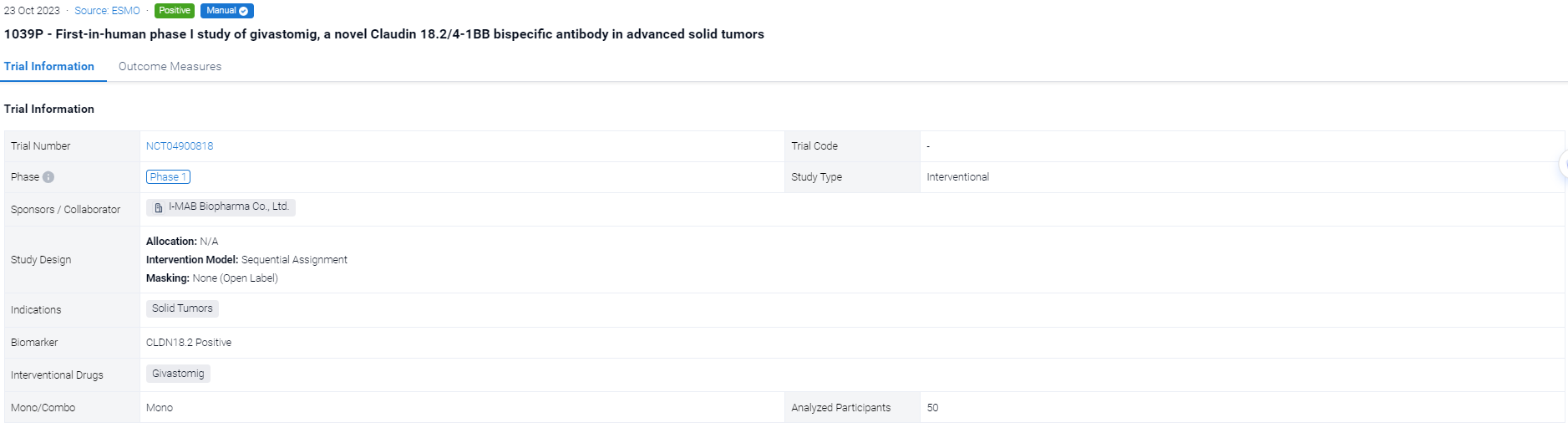
In this study, Givastomig was administered intravenously every 2 weeks (Q2W) across 8 dose levels (0.1-15mg/kg) in patients with advanced solid tumors. The Bayesian Optimal Interval design was utilized during dose escalation without CLDN18.2 preselection. Additionally, 6 CLDN18.2+ patients per dose level were enrolled at 5, 8, and 12mg/kg in parallel expansions. CLDN18.2+ is defined as ≥1% of tumor cells with ≥1+ intensity by immunohistochemistry.

The result showed that As of 18 April 2023, 50 patients were enrolled (32 in the dose escalation and 18 in the parallel expansions) with a median age of 66 years, ECOG 0/1 (34%/66%), and a median of 3 prior lines of therapy. No dose-limiting toxicity was observed up to 15 mg/kg, and maximum tolerated dose was not reached. The most common treatment-related adverse events (TRAEs) were Grade 1/2 nausea (22%), fatigue (14%), and vomiting (12%) with 7 sporadic Grade 3 TRAEs, and no Grade ≥4 TRAEs. Givastomig exhibited a linear PK at doses ≥5 mg/kg and a dose-dependent increase of s4-1BB levels reaching a plateau at doses ≥8 mg/kg. Among 18 efficacy-evaluable patients with CLDN18.2+ gastroesophageal cancer (GEC) at 5, 8, and 12mg/kg, partial response (PR) in 1 patient each at 5 and 8 mg/kg, unconfirmed PR in 2 patients at 12 mg/kg, and stable disease in 2 patients were observed. An additional PR was observed in 1 patient with head and neck cancer at 12mg/kg. CLDN18.2 expression among the responders ranged from 11% to 100%.
It can be concluded that Givastomig is well tolerated up to 15 mg/kg Q2W. Encouraging monotherapy activity was observed in heavily pre-treated GEC patients with a wide range of CLDN18.2 expression, including low CLND18.2 tumors. The PK profile, s4-1BB induction, and efficacy signal support 12 mg/kg as an optimal dose. Further development of givastomig mono- and combo- therapy in GEC and other cancers is planned.
How to Easily View the Clinical Results Using Synapse Database?
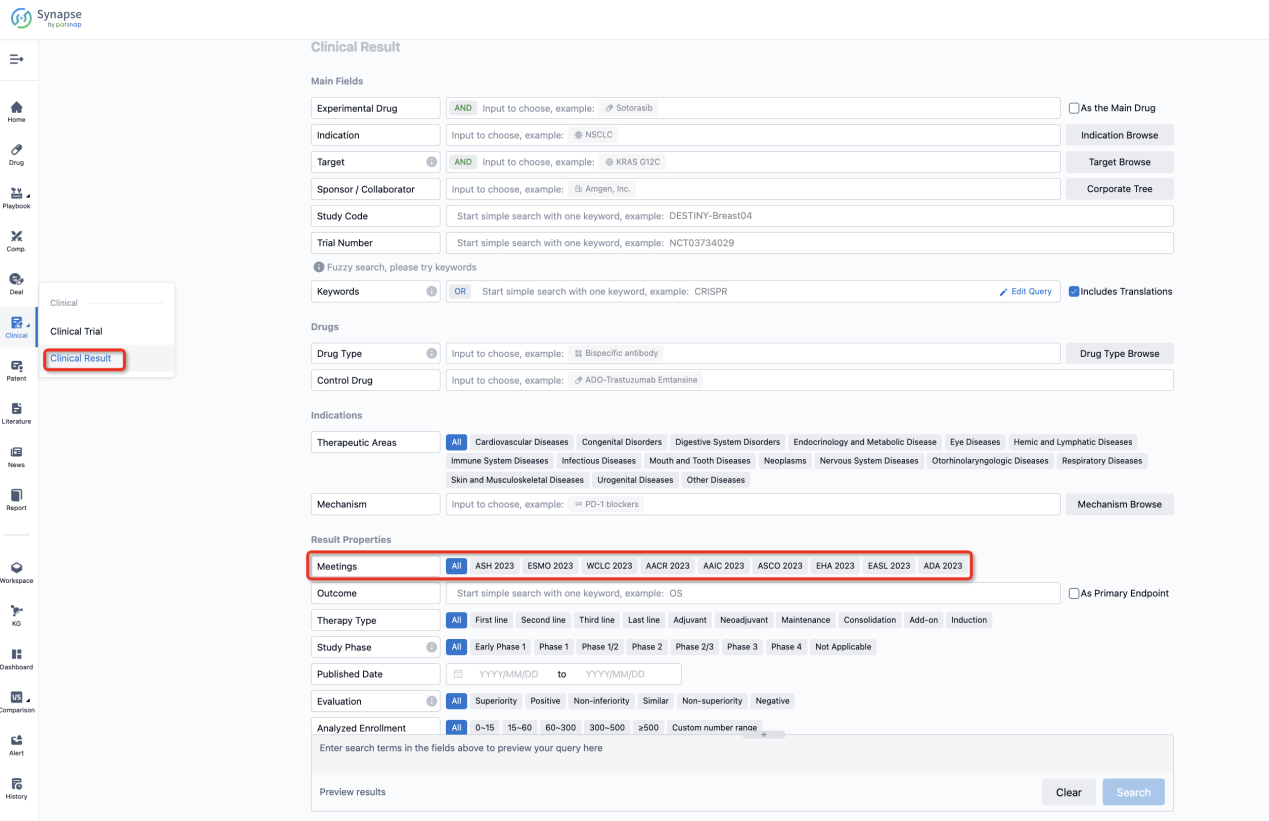
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
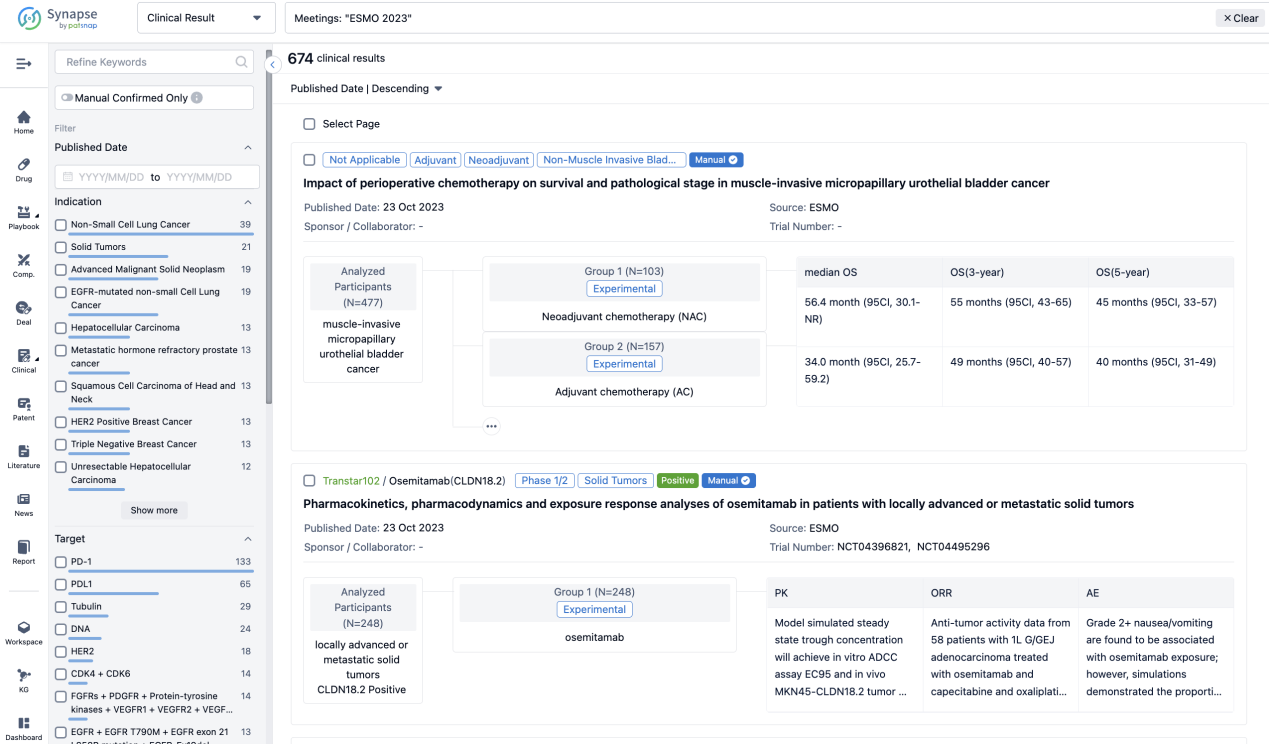
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.

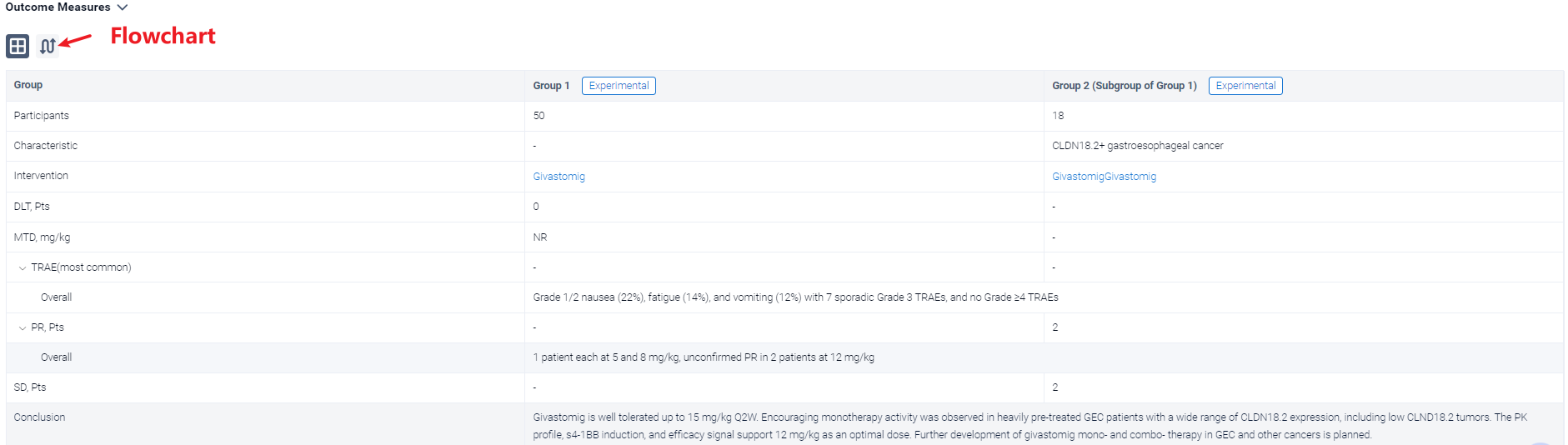
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.

Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!