Glenmark Ichnos Sciences Exhibits Early-Stage Results of Cancer Drug Candidate ISB 2001 at 2024 AACR Conference
At the American Association for Cancer Research's annual meeting in 2024, a collaborative venture known as Ichnos Glenmark Innovation, which combines the expertise of Ichnos Sciences Inc.—a worldwide, complete clinical-stage biotechnology firm focused on the creation of multispecifics™ for cancer treatment—and Glenmark Pharmaceuticals Ltd., publicly disclosed early-stage research findings for their cancer-fighting candidate, ISB 2001. This oral presentation outlined the preclinical achievements of ISB 2001, which is now undergoing a Phase I clinical study targeting relapsed and refractory multiple myeloma (r/r MM).
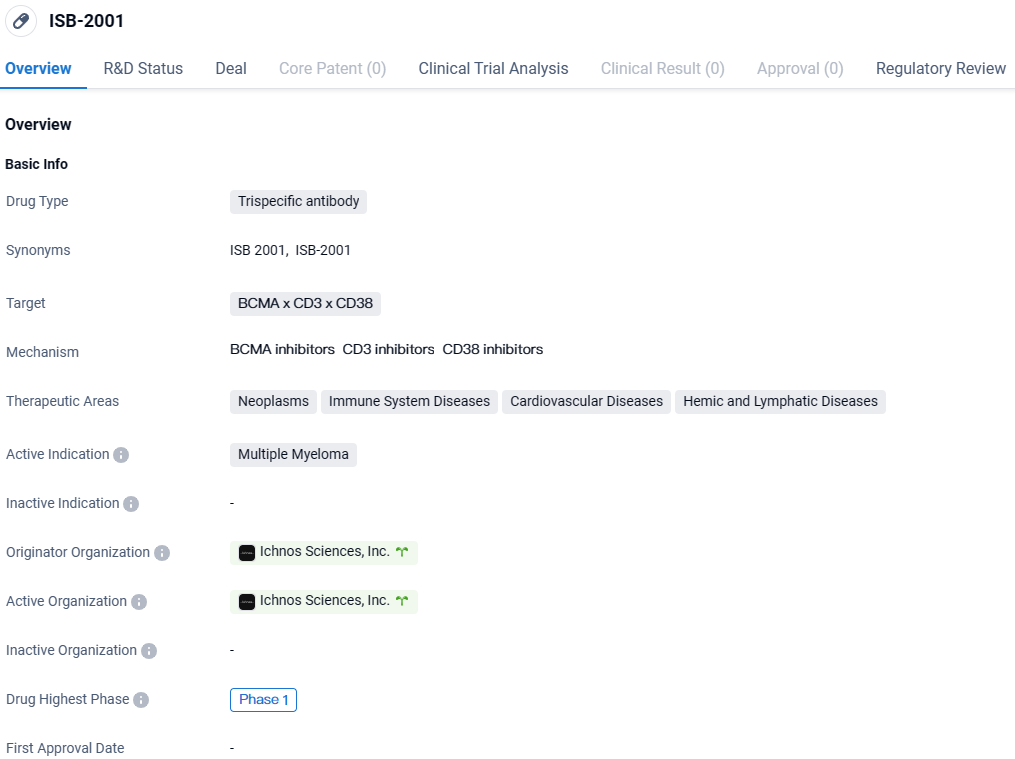
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The verbal exposition presented data on the efficacy of ISB 2001, demonstrating its potential in treating multiple myeloma (MM) within bone marrow samples taken from subjects at initial diagnosis or those enduring relapsed/refractory MM after undergoing various therapy regimens, including those who have shown resistance to therapies targeting CD38 and BCMA. This foundational research highlights the potential capacity of the ISB 2001 trispecific antibody to attack BCMA, CD38 on multiple myeloma cells, and CD3 on T lymphocytes. It underscores the commitment of IGI to contribute to the battle against resistant forms of MM.
"At the AACR conference, we are excited to discuss the early-stage results associated with ISB 2001," expressed Lida Pacaud, MD, the Chief Medical Officer at Ichnos Glenmark Innovation. "The progression of ISB 2001 is of unique importance to our team as it symbolizes the application of our state-of-the-art BEAT antibody engineering platform. This key technology underpins our innovative strategy to create novel therapies addressing hematologic malignancies and solid neoplasms."
Marked as a pioneering T cell-redirecting antibody, ISB 2001 concurrently engages BCMA and CD38 on MM cells. Crafted using the BEAT technique, this proprietary development framework ensures comprehensive adaptability and the capability to produce full-length multispecific antibodies efficiently. ISB 2001 fuses three specialized antigen-recognition domains, with distinct targets: one arm engages with the epsilon chain of CD3 on T lymphocytes, while the remaining arms attach to BCMA and CD38 on MM cells. Moreover, the antibody's Fc region has been ingeniously muted to reduce effector functions of the immune system.
ISB 2001 has the novel function of steering CD3+ T cells to eradicate cancer cells presenting varying levels of both BCMA and CD38. The dual targeting of two tumor-specific antigens affords ISB 2001 improved specificity in adhering to MM cells by leveraging increased binding strength due to enhanced avidity..
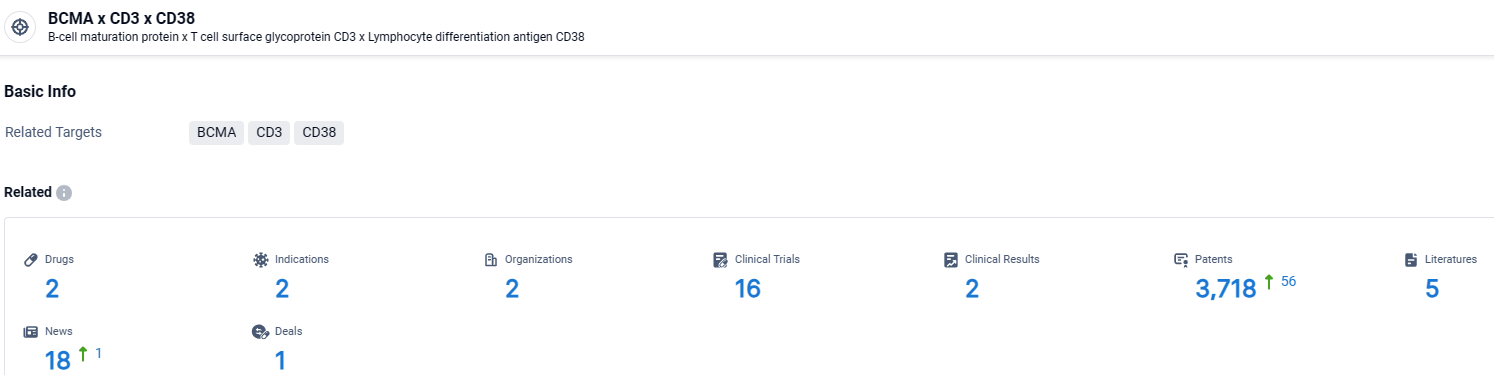
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of April 9, 2024, there are 2 investigational drugs for the BCMA and CD3 and CD38 target, including 2 indications, 2 R&D institutions involved, with related clinical trials reaching 16, and as many as 3718 patents.
ISB-2001 is a trispecific antibody drug being developed by Ichnos Sciences, Inc. for the treatment of multiple myeloma. It targets three proteins, BCMA, CD3, and CD38, and is currently in Phase 1 of clinical development. The drug has been designated as an orphan drug, indicating its potential to address a rare disease. Further research and clinical trials will be needed to determine the drug's efficacy and safety profile.