Summary of Global Clinical Trial Failures in January 2024
1.Theravance Biopharma: Yupelri (revfenacin)
Indication: Chronic Obstructive Pulmonary Disease (COPD)
Clinical Trials: Phase 4
On January 5th, Theravance Biopharma announced that the Phase IV PIFR-2 study of Yupelri (revefenacin) inhalation solution for the maintenance treatment of Chronic Obstructive Pulmonary Disease (COPD) did not meet the primary endpoint.
Yupelri is the only once-daily nebulized long-acting muscarinic antagonist (LAMA) approved in the United States for the maintenance treatment of COPD. The PIFR-2 study aimed to evaluate the improvement in lung function of Yupelri as compared to tiotropium bromide (Spiriva, a dry powder inhaler) in patients with severe to very severe COPD. The study results showed that on day 85, there was no statistically significant difference in the primary endpoint of change from baseline in trough Forced Expiratory Volume in one second (FEV1) compared to the control group. The safety and tolerability profile of Yupelri was consistent with previous studies.
2. Novartis: Ligelizumab
Indication: peanut allergy
Clinical Trials: Phase 3
On January 16th, the clinicaltrials.gov website indicated that Novartis has discontinued the Phase III clinical trial for the treatment of peanut allergy with the immunoglobulin E (IgE) monoclonal antibody Ligelizumab (study CQGE031G12301).
This study was a multicenter, randomized, double-blind, placebo-controlled clinical trial, which initially aimed to enroll 486 patients medically diagnosed with IgE-mediated peanut allergy, to evaluate the efficacy and safety of Ligelizumab (120/240mg, once a month) compared to a placebo. As of the termination date of the trial, 211 patients had been enrolled.
This termination marks the second setback encountered by Ligelizumab in the immunotherapy field. In September 2023, Novartis formally terminated the Phase III PEARL-PROVOKE study for the treatment of chronic inducible urticaria (CIU) with Ligelizumab, due to the drug not demonstrating a significant therapeutic advantage in two Phase III studies (PEARL 1 and PEARL 2) for the treatment of chronic spontaneous urticaria (CSU). Although Ligelizumab was more effective than a placebo, it was less effective than omalizumab.
3. Allakos: lirentelimab
Indication: Chronic Spontaneous Urticaria
Clinical Trials: Phase 2
On January 16th, Allakos announced that lirentelimab did not meet the primary endpoints in the Phase 2 clinical trial (ATLAS) for patients with atopic dermatitis and the Phase 2b clinical trial (MAVERICK) for patients with chronic spontaneous urticaria.
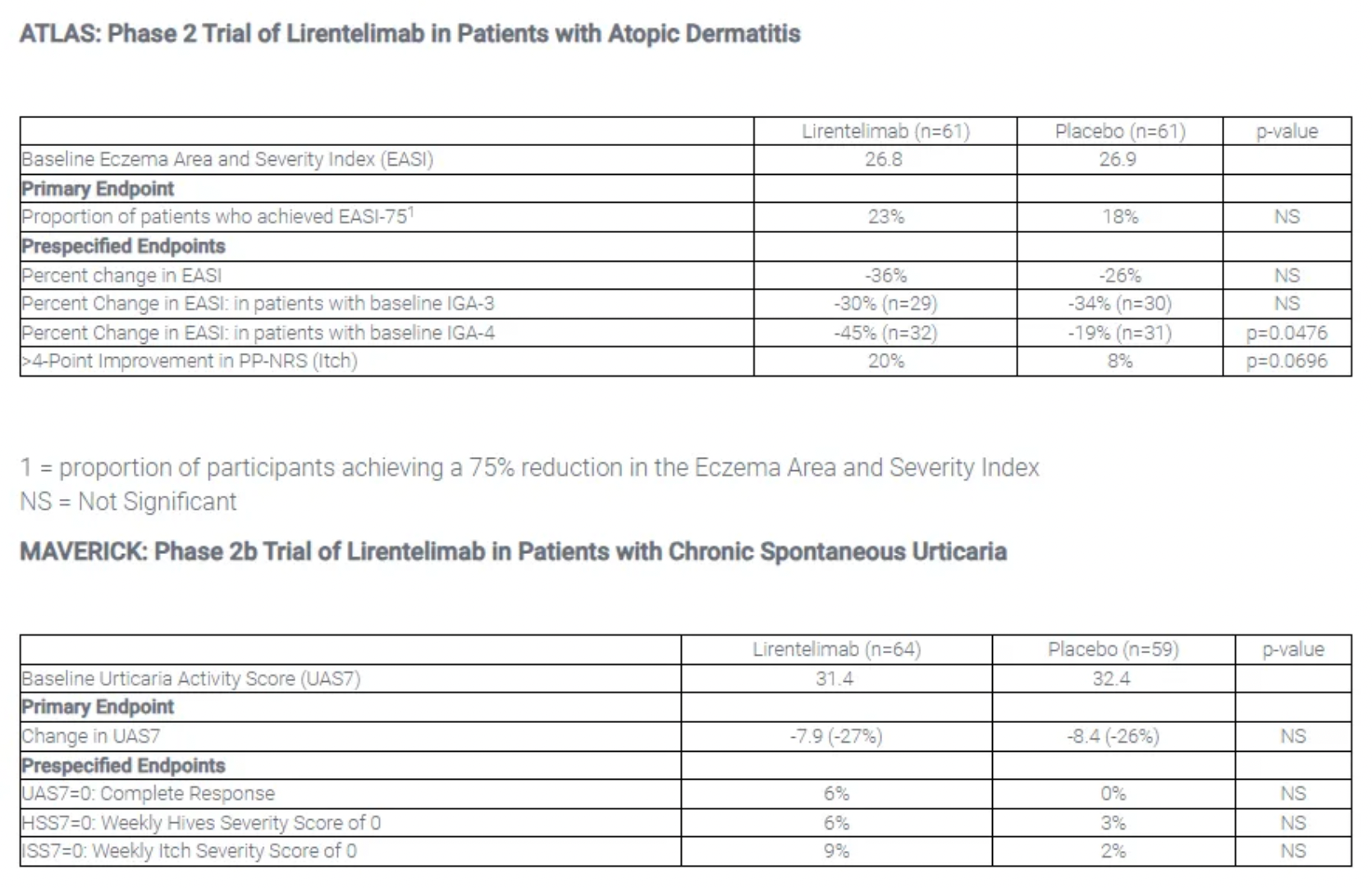
The results of the Phase 2b clinical trial (ATLAS) for the treatment of atopic dermatitis patients with lirentelimab showed that, at the primary endpoint after treatment to achieve EASI-75, 23% of patients treated with lirentelimab reached EASI-75 compared to 18% of patients treated with placebo, which was not statistically significant. In the MAVERICK trial, for the primary endpoint on the reduction of the UAS7 score, patients treated with lirentelimab saw a 27% reduction in UAS7 compared to a 26% reduction in the placebo-treated group, which also lacked statistical significance.
4. Gilead: Trodelvy® (sacituzumab-govitecan-hziy; SG)
Indication: non-small cell lung cancer (NSCLC)
Clinical Trials: Phase 3
On January 22, Gilead announced that its Trop2 ADC (antibody-drug conjugate) new drug, Trodelvy, did not meet the primary OS (overall survival) endpoint in the Phase III clinical trial EVOKE-01 for the treatment of non-small cell lung cancer (NSCLC). EVOKE-01 is assessing the efficacy of the Trop2 ADC drug Trodelvy® (sacituzumab govitecan-hziy; SG) in combination with docetaxel in patients with metastatic or advanced NSCLC who have progressed during or after treatment with platinum-based chemotherapy and checkpoint inhibitors.

Trodelvy is a Trop-2 targeting ADC monoclonal antibody drug. Trop-2 is a transmembrane glycoprotein whose high expression is associated with the occurrence of many tumors and poorer prognosis, making it a very popular target for ADC research, second only to HER2 in terms of interest. Currently, Gilead's Trop-2 ADC drug Trodelvy is the only one approved globally for the treatment of adult patients with metastatic triple-negative breast cancer (mTNBC) who have previously received at least two therapies, and for patients with locally advanced or metastatic urothelial carcinoma (UC).
5. BMS: Opdivo
Indication: Second Time in Late-Stage Kidney Cancer
Clinical Trials: Phase 3
On January 29, Bristol-Myers Squibb (BMS) announced the failure of its PD-1 inhibitor, Opdivo, in Part B of the Phase III CheckMate-914 study, as it did not provide a significant benefit in disease-free survival for patients at high risk of recurrence after local renal cell carcinoma surgery.

CheckMate-914 is a randomized, double-blind, two-part trial. In Part B, the study compared monotherapy with Opdivo to a placebo. The trial was designed to assess its use as an adjuvant treatment for renal cell carcinoma (RCC) patients who have undergone complete or partial nephrectomy and are at intermediate or high risk of recurrence. After a median follow-up of 27 months, Opdivo failed to demonstrate a significant improvement in disease-free survival (DFS) as compared to placebo, with a hazard ratio of 0.87 and a p-value of 0.3952 based on an assessment by blinded independent central review (BICR).
The 12-month DFS probability for the Opdivo group was 83.3%, compared with 78.2% for the placebo group. At 18 months, these estimates changed only slightly to 78.4% and 75.4%, respectively. At the time of analysis, the median DFS for both treatment groups had not been reached.
6. Laekna: afuresertib
Indication: platinum-resistant ovarian cancer (PROC)
Clinical Trials: Phase 2
On January 29, Laekna Pharma announced the top-line data from the PROFECTA-II study for the combination of afuresertib with paclitaxel in the treatment of platinum-resistant ovarian cancer (PROC).
The PROFECTA-II study (NCT04374630) is a randomized, open-label, active-controlled phase II clinical trial evaluating the efficacy and safety of the combination of afuresertib and paclitaxel compared to paclitaxel alone in female patients with platinum-resistant ovarian cancer (PROC). The study enrolled 150 patients randomly across the United States and China into both the experimental and control groups. The primary endpoint was progression-free survival as assessed by the researchers, while secondary endpoints included overall survival, objective response rate, and duration of response. Trial results indicated that the combination treatment of afuresertib and paclitaxel reduced the risk of disease progression or death (progression-free survival, PFS), with an HR of 0.744 (95% CI: 0.502-1.102); however, the results were not statistically significant.
7. Novartis: sabatolimab
Indication: intermediate, high, or very high-risk myelodysplastic syndromes (MDS) or chronic myelomonocytic leukemia-2 (CMML-2)
Clinical Trials: Phase 3
On January 31, in its 2023 financial report, Novartis provided updates on its pipeline progress, revealing that the phase III STIMULUS MDS2 study of its TIM-3 monoclonal antibody, sabatolimab (MBG453), in combination therapy for intermediate, high, or very high-risk myelodysplastic syndromes (MDS) or chronic myelomonocytic leukemia-2 (CMML-2), did not meet its primary endpoint of overall survival (OS). As a result, Novartis has removed it from its pipeline. Moreover, Novartis has also decided to discontinue the development of the BTK inhibitor remibrutinib for the treatment of Sjögren’s syndrome.
TIM-3 is a receptor protein belonging to the T cell immunoglobulin and mucin domain (TIM) family that is expressed on the surfaces of T cells, regulatory T cells (Tregs), and innate immune cells (including dendritic cells, natural killer cells, and monocytes). Previously, Novartis had posited that sabatolimab could act by inhibiting the function of the TIM-3 receptor, thereby targeting both myeloid leukemia cells and immune cells simultaneously. This inhibition was hoped not only to kill cancer cells but also potentially enhance the vigor of immune cells.