Glycyrrhetinic Acid Unveiled: A Detailed Overview of its Revolutionary R&D Breakthroughs
Glycyrrhetinic Acid's R&D Progress
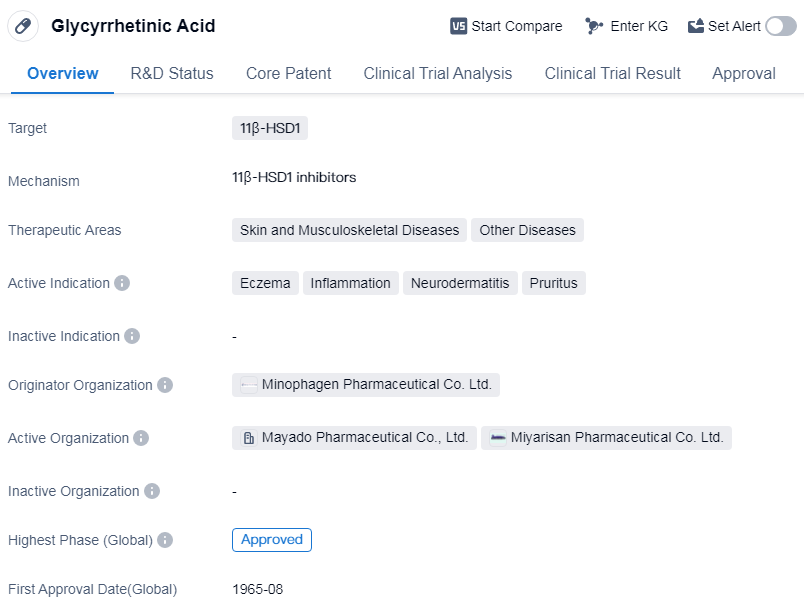
Glycyrrhetinic Acid is a small molecule drug that targets 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1). It is primarily used in the treatment of skin and musculoskeletal diseases, as well as other diseases. The active indications for this drug include eczema, inflammation, neurodermatitis, and pruritus.
The drug was first approved in Japan in August 1965, making it one of the oldest drugs on the market. It is important to note that the information provided does not specify the current approval status in other countries or regions.
Glycyrrhetinic Acid is developed by Minophagen Pharmaceutical Co. Ltd., an originator organization in the pharmaceutical industry. As an approved drug, it has successfully completed the highest phase of clinical trials, indicating its safety and efficacy.
The therapeutic areas of this drug suggest its potential in treating various skin and musculoskeletal diseases, which are common conditions affecting a significant portion of the population. Eczema, inflammation, neurodermatitis, and pruritus are all conditions that cause discomfort and can significantly impact the life quality of patients.
The drug's target, 11β-HSD1, is an enzyme involved in the metabolism of cortisol, a hormone that plays a crucial role in regulating inflammation and immune responses. By targeting this enzyme, Glycyrrhetinic Acid may help modulate the inflammatory processes, providing relief for patients with skin and musculoskeletal diseases.
Given the drug's long history since its first approval in Japan, it is likely that Glycyrrhetinic Acid has been extensively studied and its safety profile well-established. However, without additional information, it is difficult to assess the drug's current market presence, potential side effects, or any ongoing research and development efforts.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Glycyrrhetinic Acid: 11β-HSD1 Inhibitors
11β-HSD1 inhibitors are a type of drugs that target and inhibit the enzyme 11β-HSD1. This enzyme is involved in the conversion of inactive cortisone to active cortisol, a hormone that plays a crucial role in regulating various physiological processes, including metabolism, immune response, and inflammation.
From a biomedical perspective, 11β-HSD1 inhibitors are designed to modulate the activity of this enzyme, thereby reducing the production of cortisol. By inhibiting 11β-HSD1, these drugs can potentially be used to treat conditions associated with excessive cortisol levels, such as metabolic disorders (e.g., obesity, diabetes), immune disorders, and certain inflammatory diseases.
The inhibition of 11β-HSD1 can help restore the balance of cortisol, which may have therapeutic benefits in certain pathological conditions. These inhibitors can be developed as small molecules or biologics, depending on their specific chemical structure and mechanism of action.
It's important to note that the use of 11β-HSD1 inhibitors as therapeutic agents is still under investigation, and their efficacy and safety profiles are being evaluated through preclinical and clinical studies.
Drug Target R&D Trends for Glycyrrhetinic Acid
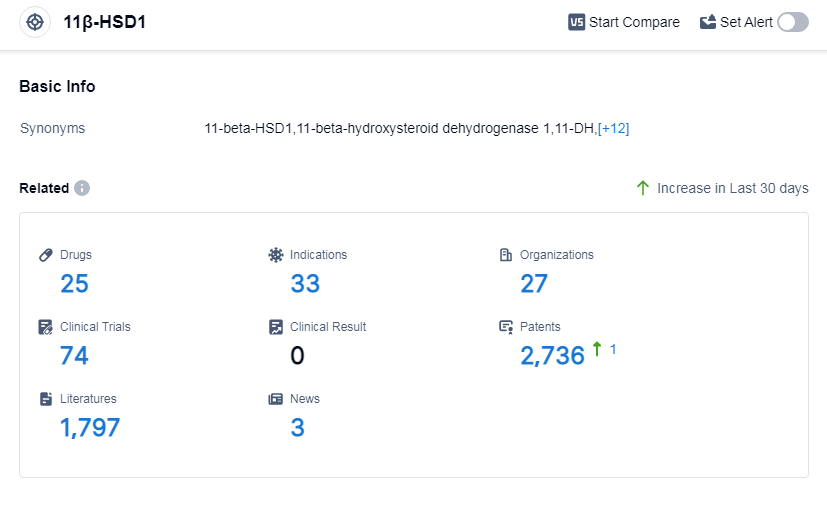
According to Patsnap Synapse, as of 14 Sep 2023, there are a total of 25 11β-HSD1 drugs worldwide, from 27 organizations, covering 33 indications, and conducting 74 clinical trials.
Based on the analysis of the provided data, the current competitive landscape of target 11β-HSD1 shows that several companies are actively involved in the research and development of drugs. Sino Biopharmaceutical Ltd., Rohto Pharmaceutical Co., Ltd., Santen Pharmaceutical Co., Ltd., and Miyarisan Pharmaceutical Co. Ltd. have all received approval for drugs targeting 11β-HSD1. Sparrow Pharmaceuticals, Inc. and Pinotbio are also making progress with drugs in phase 2 and preclinical development stages.
The approved drugs targeting 11β-HSD1 have indications ranging from eczema to hepatitis, demonstrating the potential of this target in treating various conditions. Additionally, small molecule drugs are the most rapidly progressing drug type under this target.
China is leading in the development of drugs targeting 11β-HSD1, followed by Japan and the United States. Other countries and locations, including the European Union, United Kingdom, South Korea, and Germany, are also actively involved in the development of drugs targeting 11β-HSD1.
Overall, the analysis suggests a competitive landscape with multiple companies and countries actively pursuing the development of drugs targeting 11β-HSD1. The future development of this target holds promise for the treatment of various indications and the advancement of small molecule drugs.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In conclusion, Glycyrrhetinic Acid is a small molecule drug developed by Minophagen Pharmaceutical Co. Ltd. It targets 11β-HSD1 and is primarily used in the treatment of skin and musculoskeletal diseases, as well as other diseases. With active indications including eczema, inflammation, neurodermatitis, and pruritus, this drug has the potential to provide relief for patients suffering from these conditions. Its approval in Japan in 1965 indicates its long-standing presence in the market, although further information is needed to fully assess its current status and potential.