GPN Vaccines Reports Positive Early-Phase Safety and Immune Response for Gamma-PN Vaccine
GPN Vaccines Ltd, an enterprise in the clinical development phase specializing in biotechnological research, is proud to report encouraging findings relating to safety and immune response from its initial clinical evaluation of Gamma-PN. This investigational vaccine, designed as an unadjuvanted, whole-cell pneumococcal formulation, targets the prevention of severe ailments such as pneumonia, septicaemia, and meningitis caused by Streptococcus pneumoniae. The study encompassed a demographic of adult participants ranging from 50 to 69 years of age.
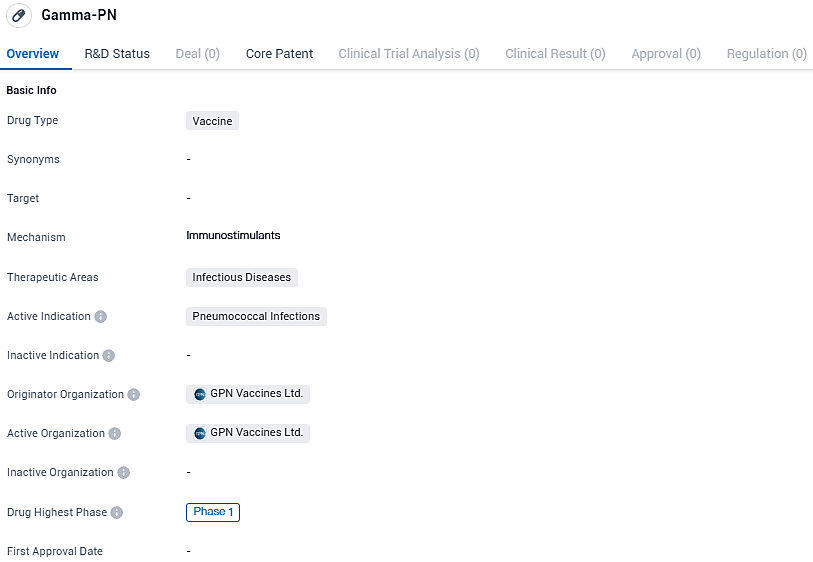
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In a meticulously designed, placebo-controlled, double-dummy trial involving 120 subjects, we administered varying amounts of Gamma-PN (50, 250, or 1000 micrograms in doses, with each stratum encompassing 30 individuals); licensed pneumococcal vaccinations (either Pneumovax-23® or Prevenar 13®, with 9 subjects per vaccine group); or a placebo for comparison.
The study successfully achieved its main goal related to safety, confirming that Gamma-PN's safety profile warrants progression to subsequent phases of research. Notably, there were no severe adverse reactions reported post Gamma-PN injection, and its systemic acceptance was deemed outstanding. Reactions at the injection site were generally minor, consisting of mild-to-moderate redness and swelling, which lasted for a brief period (usually 1 to 2 days), with both the likelihood and severity proportionate to the dosage administered. Both local and systemic responses encountered after the second administration of Gamma-PN mirrored the reactions observed following the initial dose.
The investigation included an immunological evaluation, analyzing the serum IgG responses post-vaccination with Gamma-PN. These analyses were executed through an ELISA procedure, established and conducted in accordance with GLP standards by an independent laboratory. The outcomes indicate that Gamma-PN elicited a quantifiable dose-responsive increase in serum IgG concentration subsequent to each inoculation. The observed neutrophil activation in subjects receiving Gamma-PN corroborated the anticipated activation of innate immune mechanisms by the vaccine.
Additionally, GPN Vaccines is carrying out further immunological examinations on collected serum and peripheral blood mononuclear cells. Part of this additional research focuses on analyzing opsonophagocytic antibodies that target various Streptococcus pneumoniae serotypes. These include serotypes currently encompassed by sanctioned pneumococcal vaccines as well as those that are not. Findings from these studies are anticipated and will be made publicly available upon completion.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
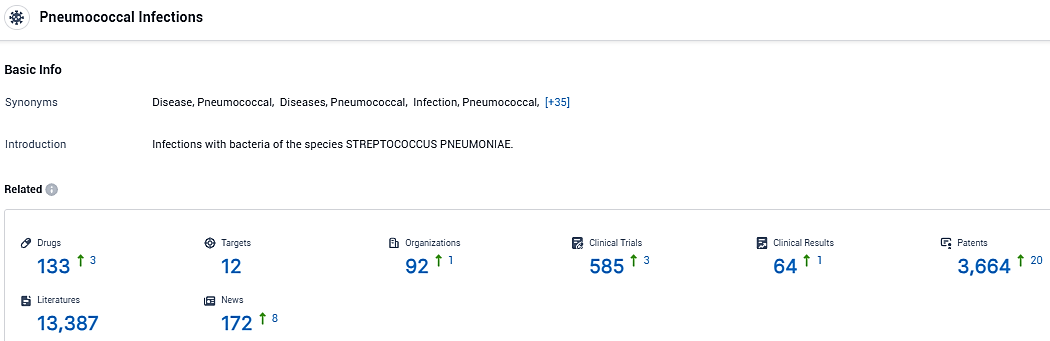
According to the data provided by the Synapse Database, As of January 9, 2024, there are 133 investigational drugs for Pneumococcal Infections, including 12 targets, 92 R&D institutions involved, with related clinical trials reaching 585, and as many as 3664 patents.
Gamma-PN is a vaccine being developed by GPN Vaccines Ltd. for the treatment of pneumococcal infections. It is currently in Phase 1 of clinical trials, indicating that it has shown promise in preclinical testing and is now being evaluated for safety and dosage in human volunteers. The successful development of Gamma-PN could potentially provide an effective preventive measure against pneumococcal infections, benefiting individuals at risk of these infectious diseases.