Genascence Corp. begins Phase 1b trial for knee arthritis gene therapy GNSC-001
Genascence Corporation, an innovative biotech entity at the clinical phase, is transforming the management of widespread musculoskeletal conditions using gene therapeutic methods. The company has recently declared the commencement of its early-stage Phase 1b clinical study, employing their novel treatment, GNSC-001, specifically targeting the alleviation of symptoms in patients afflicted with knee osteoarthritis.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Recruitment for the investigation is currently underway at a network of ten clinical study sites across the U.S. Around 50 subjects are expected to join the trial, which is anticipated to reach full enrollment by the beginning of 2024.
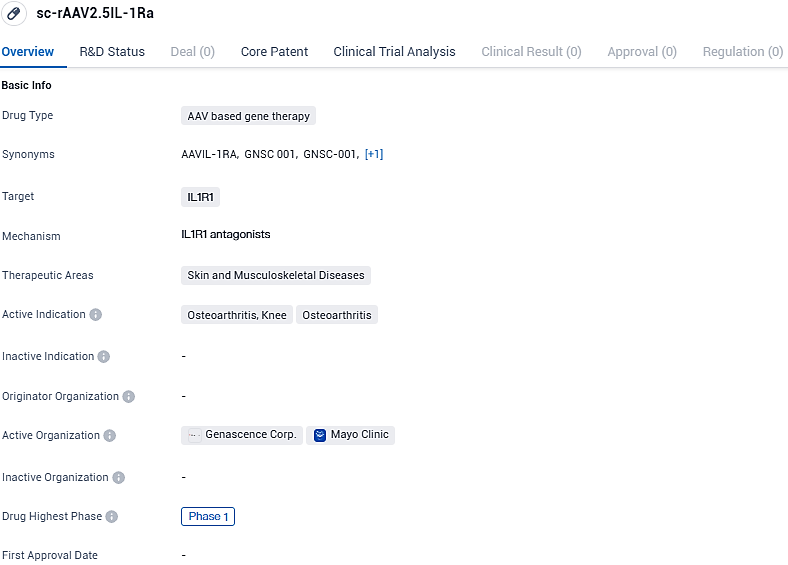
GNSC-001 represents a novel genetic therapeutic approach – it is a recombinant adeno-associated virus (AAV) vector engineered to express an enhanced version of IL-1Ra, a protein that naturally inhibits the activity of interleukin-1 (IL-1). IL-1 plays a crucial role in the development of osteoarthritis (OA), contributing to inflammation, pain in the joints, and damage to cartilage. GNSC-001 aims to deliver persistent suppression of IL-1 with a single injection directly into the joint affected by OA.
"Individuals with osteoarthritis experience debilitating long-term pain and impaired mobility, yet we currently lack therapies that can slow down the progression of the disease," stated Thomas Chalberg, Ph.D., the innovator behind Genascence and its CEO. "Our team is thrilled to see the Phase 1B DONATELLO trial actively progressing in ten locations nationwide, marking an important move forward in developing the first gene-based treatment for widespread diseases such as OA."
"The GNSC-001 gene therapy holds promise to revolutionize how OA is treated," remarked Lachy McLean, M.D., Ph.D., and chief medical officer at Genascence. "We are eager to unveil the preliminary data from the DONATELLO study by the end of 2024 and to continue propelling our clinical efforts to deliver this innovative treatment solution to patients with the utmost urgency."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
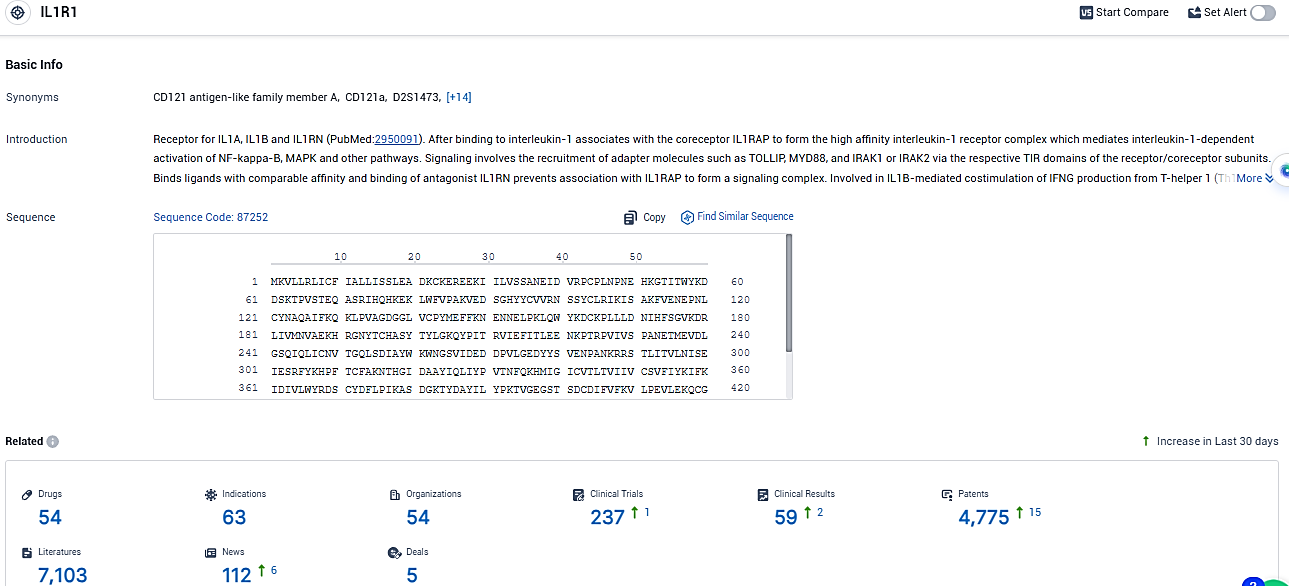
According to the data provided by the Synapse Database, As of January 9, 2024, there are 54 investigational drugs for the IL1R1 target, including 63 indications, 54 R&D institutions involved, with related clinical trials reaching 237, and as many as 4775 patents.
GNSC-001 is an AAV-based gene therapy that targets IL1R1 for the treatment of skin and musculoskeletal diseases, specifically osteoarthritis of the knee. It is currently in Phase 1 of clinical development, focusing on safety and dosage evaluation. The drug's mechanism of action involves delivering a therapeutic gene that produces IL-1Ra, an antagonist to IL1R1, to reduce inflammation.