GSK reveals positive results in phase III DREAMM-7 study comparing Blenrep for relapsed or refractory multiple myeloma
GSK plc has publicized encouraging initial findings from a scheduled mid-course effectiveness review of the DREAMM-7 phase III study. This research gauges the performance of belantamab mafodotin when used as a secondary option for treating recurring or resistant multiple myeloma.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The study achieved its main goal of PFS and demonstrated that belantamab mafodotin in conjunction with bortezomib and dexamethasone significantly enhanced the duration to disease progression or death compared to daratumumab along with BorDex, which is the existing standard care for relapsed/refractory multiple myeloma. A substantial and clinically significant OS trend with nominal p value < 0.0005 was noted at the point of this analysis, with continuous follow up for OS.
Hesham Abdullah, the Senior Vice President and Global Head of Oncology, R&D, at GSK, commented, "We find it particularly promising that belantamab mafodotin, when used with BorDex, could cater to the high unfulfilled demand in relapsed/refractory multiple myeloma, with the fact of it being compared directly with the daratumumab-based standard of care regimen.”
The DREAMM clinical research scheme is persistently examining the potential of belantamab mafodotin in the initial lines of treatment and when blended with innovative therapies and standard of care treatments. This includes the ongoing direct comparison phase III DREAMM-8 research, studying belantamab mafodotin combined with pomalidomide and dexamethasone versus bortezomib mixed with pomalidomide and dexamethasone. The data from DREAMM-8 is anticipated to be available in the latter half of 2024.
Blenrep is a drug that combines an antibody with a humanized B-cell maturation antigen monoclonal antibody connected to the cytotoxic agent auristatin F via a non-cleavable linker. This drug linker technology is licensed by Seagen Inc., and the monoclonal antibody is produced via POTELLIGENT Technology, licensed by BioWa Inc., part of the Kyowa Kirin Group.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
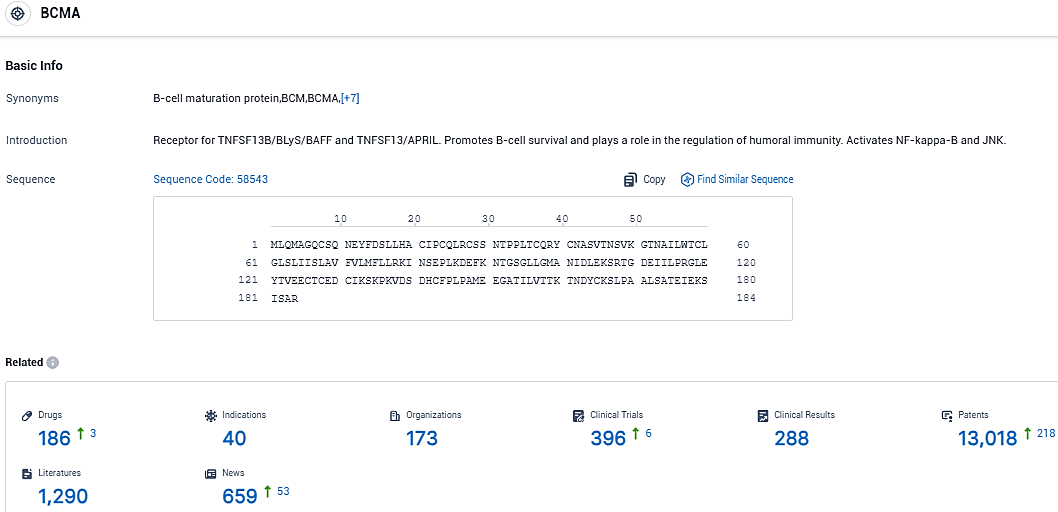
According to the data provided by the Synapse Database, As of November 30, 2023, there are 186 investigational drugs for the BCMA target, including 40 indications, 173 R&D institutions involved, with related clinical trials reaching 396, and as many as 13018 patents.
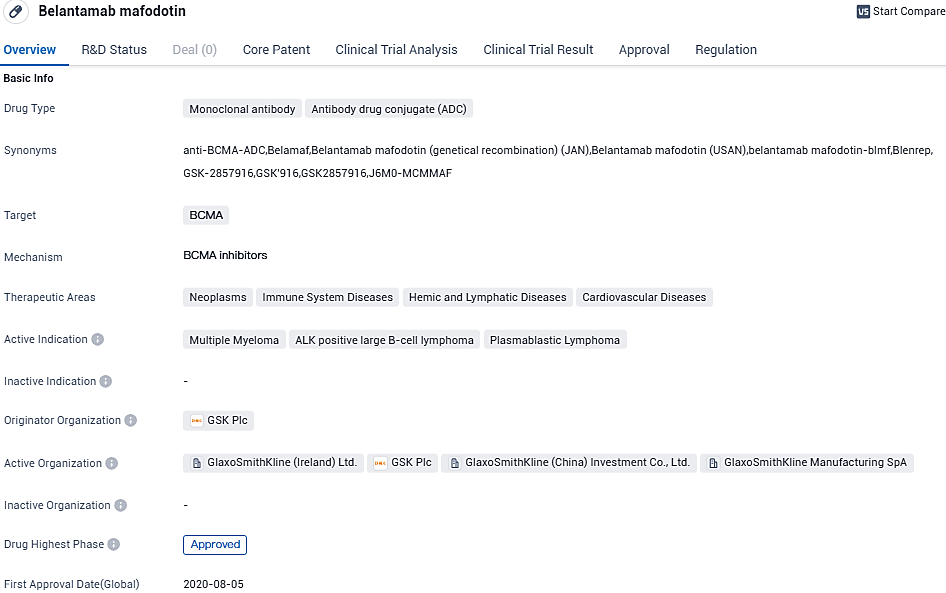
belantamab mafodotin is a monoclonal antibody and antibody drug conjugate that targets BCMA. The drug has reached the highest phase of approval globally and received its first approval in the United States in August 2020. It has been granted accelerated approval and orphan drug status, highlighting its potential to address unmet medical needs and rare disease. Refer to the Blenrep EMA Reference Information for a full list of adverse events and the complete important safety information in the EU.