Hanmi Pharmaceutical and Beijing Hanmi Progress BH3120 Cancer Immunotherapy Trials
Recent advancements in the clinical investigation of BH3120, a novel immunotherapy created in collaboration between Hanmi Pharmaceutical and Beijing Hanmi Pharmaceutical, were showcased at a prominent international academic meeting, garnering considerable interest within the immunotherapy sector. On November 25, Hanmi revealed that it had shared findings and clinical developments regarding BH3120 during a poster session at the Society for Immunotherapy of Cancer (SITC) conference, which took place in Houston, USA, from November 6 to 10.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
BH3120 represents an innovative anticancer treatment developed using Hanmi's exclusive dual antibody technology known as "Pentambody." This advanced platform allows one antibody to target two separate sites simultaneously, facilitating a focused anticancer effect by directly attacking cancer cells and activating immune cells to boost the outcomes of immunotherapy.
Designed to engage PD-L1 found on tumor cells and 4-1BB on immune cells, BH3120 functions as a connector that enhances the ability of immune cells to recognize and eliminate cancer cells.
Although other candidates targeting 4-1BB have encountered challenges in anticancer effectiveness or safety, preclinical research on BH3120 has shown considerable anticancer activity and a distinctive separation of immune responses between the tumor microenvironment (TME) and healthy tissues. This novel mechanism positions BH3120 as a promising advancement in crafting safer and more effective anticancer treatments.
During the SITC presentation, Hanmi shared insights on the foundational aspects, design, and ongoing clinical development of BH3120. At present, a global Phase 1 trial is being conducted in South Korea and the U.S., examining the safety and tolerance of BH3120 when used alone in patients with advanced or metastatic solid tumors.
The Phase 1 trial has successfully progressed through cohort 3 (1 mg/kg) of the dose escalation section, with no observed dose-limiting toxicities (DLT) or serious adverse reactions rated grade 3 or above thus far.
Dr. Dong-wan Kim, director of the Clinical Trials Center at Seoul National University Hospital (Hemato-Oncology Department) and the principal investigator for BH3120's Phase 1 trial, stated, “Initiating the Phase 1 clinical trial for BH3120 is a vital move in confirming the promise of this novel immunotherapy. We are hopeful for favorable results.” He further expressed, “We aspire that ongoing research will confirm BH3120 as an effective and safe choice for treating various cancer forms, minimizing the side effects typically seen with current immunotherapies.”
Simultaneously, Hanmi is initiating another Phase 1 trial to evaluate the safety and efficacy of BH3120 in conjunction with MSD’s (Merck & Co., Inc., Rahway, NJ, USA) anti-PD-1 therapy, KEYTRUDA® (pembrolizumab), in patients with advanced or metastatic solid tumors.
In September, Hanmi received authorization from both the Korean Ministry of Food and Drug Safety and the U.S. Food and Drug Administration (FDA) to amend the Phase 1 trial protocol to include the evaluation of BH3120 in combination with KEYTRUDA. Comprehensive clinical development is anticipated to begin in early next year, with Hanmi acting as the leading sponsor and overseeing the trial, while MSD will provide KEYTRUDA for the study.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
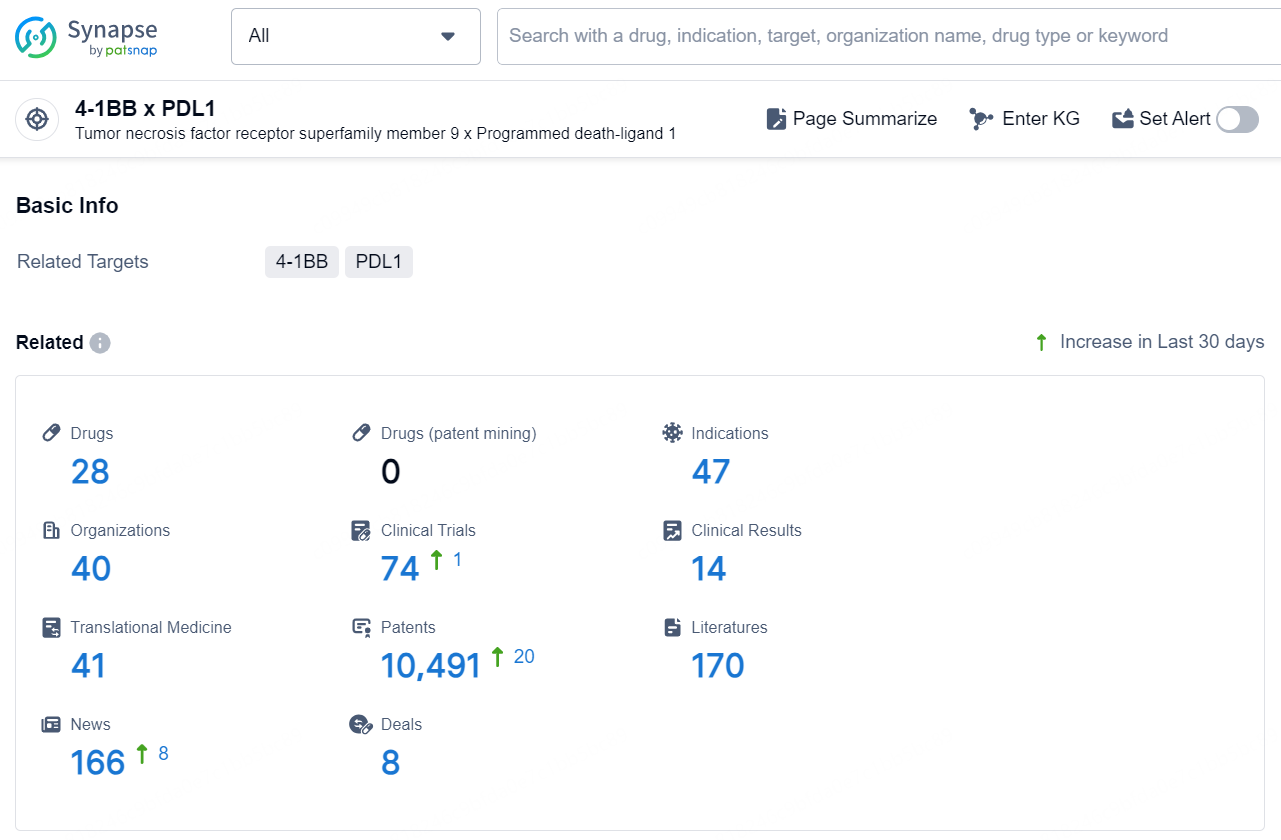
According to the data provided by the Synapse Database, As of December 4, 2024, there are 28 investigational drugs for the 4-1BB x PDL1 target, including 47 indications, 40 R&D institutions involved, with related clinical trials reaching 74, and as many as 10491 patents.
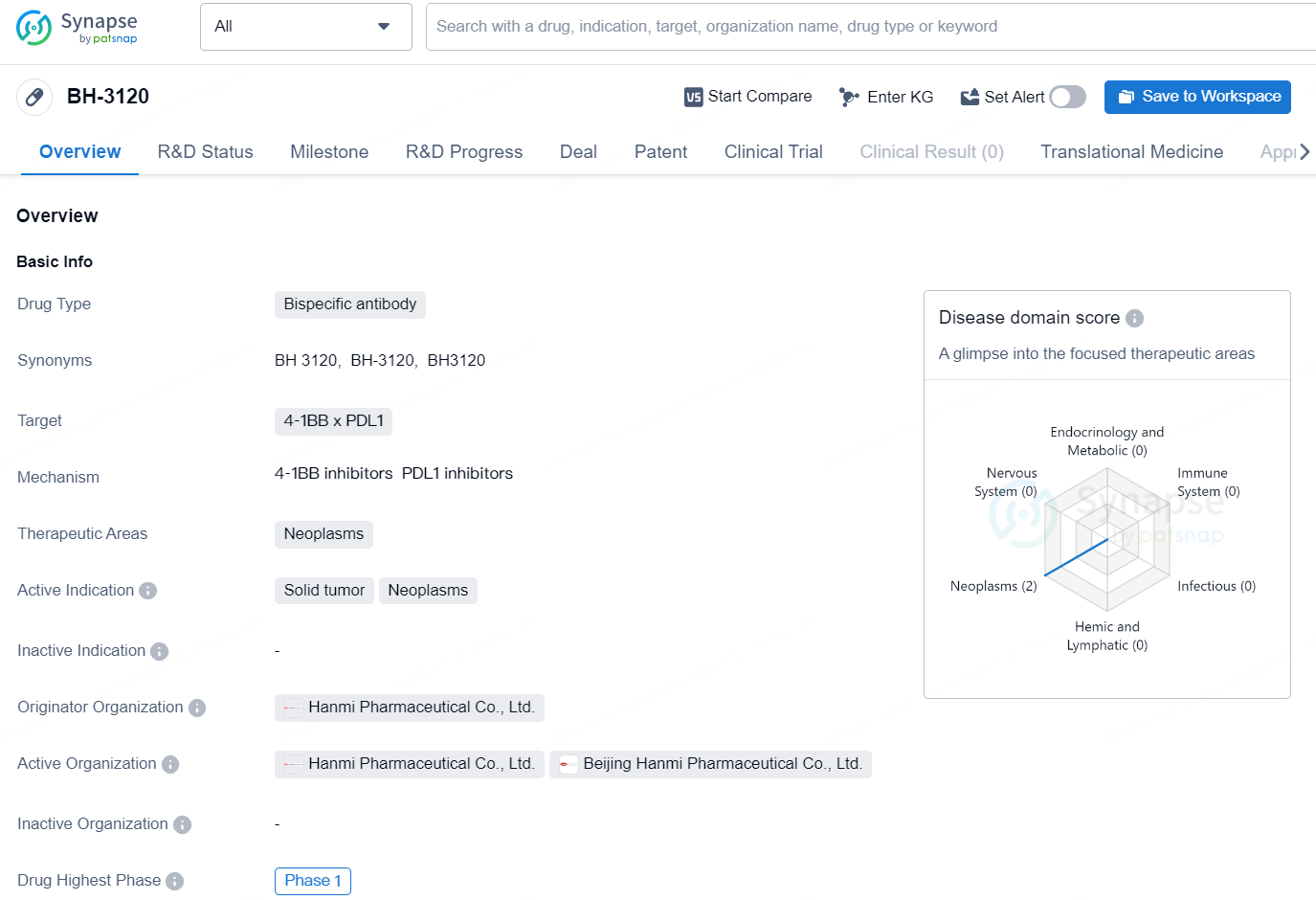
BH-3120 is a bispecific antibody drug developed by Hanmi Pharmaceutical Co., Ltd. It is designed to target the 4-1BB and PDL1 proteins and is intended for use in the treatment of neoplasms, specifically solid tumors. The drug is currently in the Phase 1 stage of development globally, indicating that it is being tested for its safety and efficacy in human subjects. In China, the highest phase of development for BH-3120 is preclinical, suggesting that it has not yet progressed to human trials in this region.