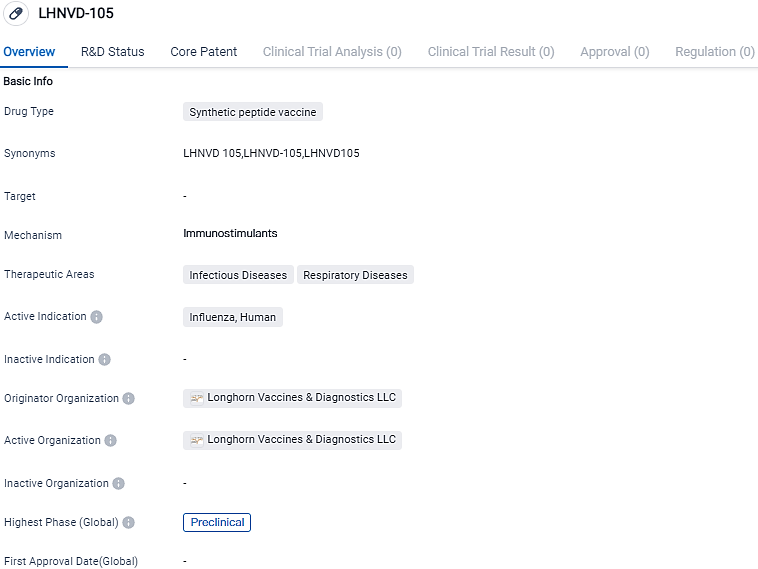
New research shows Longhorn Vaccines and Diagnostics' Universal Flu Vaccine, LHNVD-105, triggers strong, durable immunity in lab animals
Longhorn Vaccines and Diagnostics LLC a "'One Health" enterprise involved in creating vaccines and diagnostic tools for global healthcare and zoonotic diseases, revealed fresh data highlighting the possible advantages of LHNVD-105, their universal influenza vaccine under development.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The research has displayed that LHNVD-105 prompts extensive and long-lasting immunity in mice against multiple human, bird, and pig influenza viruses. The information was released in one of the latest issues of the peer-reviewed Vaccines journal.
LHNVD-105 consists of complex peptides that are not linked, developed with a safe and potent adjuvant, the Army Liposome formula. The target of this is a variety of influenza antigens preserved from the hemagglutinin, neuraminidase, and matrix proteins.
The origin of influenza strains can be wild water birds and poultry, then pass on to pigs—serving as a channel for virus transmission—and then to humans, this could lead to another pandemic if outbreaks are not monitored. By merging multiple influenza strains into one multi-epitope peptide vaccination and formulating it with ALFQ, LHNVD-105 delivers an efficient super-seasonal vaccine that is cost-effective and readily scalable. This benefits public health by bringing it nearer to a practical approach for a global influenza vaccine and readiness for pandemics.
Longhorn Vaccines & Diagnostics CEO Gerald W. Fischer, MD, said, "The previously published research on LHNVD-105 indicated that an ALFQ-adjuvanted composite influenza peptide vaccine, which includes various highly preserved antigens of HA, NA, and matrix, triggered a strong and balanced immune response.
The Vaccines study builds on these results by demonstrating the lockdown of the immune response and the range of influenza strains that the vaccine can shield, including human and avian viruses. The effectiveness of LHNVD-105 will continue to be tested as we seek a global solution to influenza prevention."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
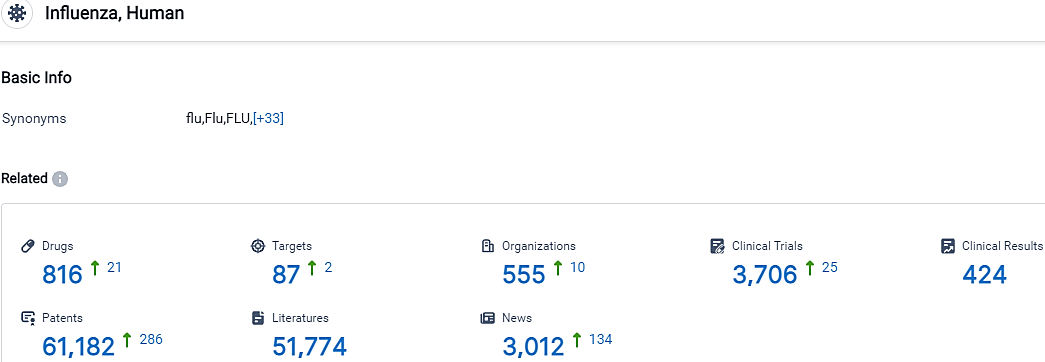
According to the data provided by the Synapse Database, As of October 5, 2023, there are 816 investigational drugs for the Influenza, including 87 targets, 555 R&D institutions involved, with related clinical trials reaching 3706,and as many as 61182 patents.
LHNVD-105, an artificial peptide vaccine, is under development by Longhorn Vaccines & Diagnostics LLC, aimed at combating human influenza. Despite being in its preliminary development stages, it shows potential as a prophylactic means against this contagious respiratory illness. Further exploration and clinical experiments are required to finalize its safety and effectiveness before it can become accessible for general use.