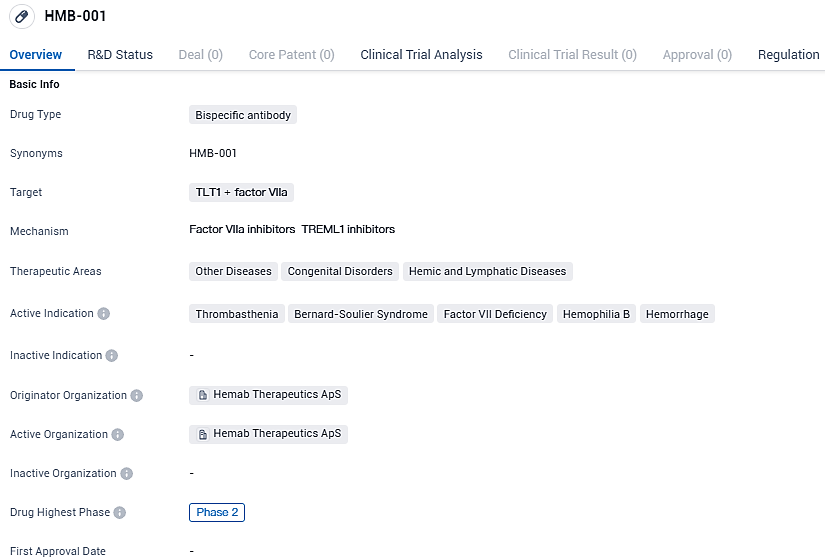
Hemab Therapeutics has begun dosing the first participant in a Phase 2 trial to evaluate the effectiveness of HMB-001 for Glanzmann's Thrombasthenia
Hemab Therapeutics, an innovative biotech firm leading the charge in crafting groundbreaking preventive treatments for critical and frequently neglected coagulation and thrombosis ailments, has disclosed the successful conclusion of the initial phase, which focused on the single-dose escalation, of their two-part clinical trial. The company is now advancing to the second phase, which will examine the effects of repeated dose increments, in the ongoing Phase 1/2 trial evaluating HMB-001 in patients with Glanzmann Thrombasthenia—this rare blood condition leads to intense and dangerous hemorrhagic incidents.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The firm has publicized that Hemab's experimental drug application for HMB-001 targeting Glanzmann Thrombasthenia (GT) has obtained the green light from the U.S. Food and Drug Administration, paving the way for patient recruiting within the United States. The initial segment of the clinical trial, which is Phase 1, reached completion in the UK. Phase 2 is set to broaden its reach by incorporating multiple new locations throughout Europe and the United States.
Furthermore, the FDA has endowed HMB-001 with the Fast Track status, acknowledging the critical and pressing demand for novel therapies addressing GT. This designation allows Hemab to engage in more regular dialogues with the FDA, thereby expediting the progression of HMB-001.
Dr. Benny Sorensen, MD, PhD, and CEO of Hemab, expressed concern for individuals afflicted with Glanzmann Thrombasthenia, as they struggle with recurrent, and sometimes severe, hemorrhages which can pose a severe threat to their health and substantially diminish their life quality. He emphasized that there is an absence of preventative treatments currently available that could effectively mitigate or halt such episodes of bleeding.
The ongoing Phase 1/2 clinical trial is designed to assess the safety, tolerability, pharmacodynamics, and pharmacokinetics of HMB-001, in addition to initial effectiveness indicators that will be determined by observing alterations in bleeding occurrence.
This comprehensive study encompasses three distinct segments: Part A, which involves a single ascending dose; Part B, multiple ascending doses; and Part C, which involves extended dosing. Hemab anticipates disclosing results from the first Phase 1 segment concerning the single ascending dose at a major international scientific conference slated for early 2024.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
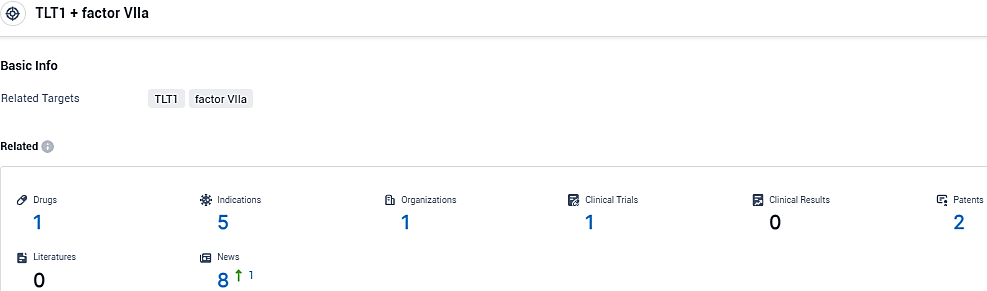
According to the data provided by the Synapse Database, As of December 17, 2023, there are 1 investigational drugs for the TLT1 and factor VIIa target, including 5 indications, 1 R&D institutions involved, with related clinical trials reaching 1, and as many as 2 patents.
HMB-001 is bispecific antibody that binds and stabilizes endogenous factor VIIa with one antibody arm and binds to TLT-1 on activated platelets with the other arm. HMB-001 is designed to be a first-in-class prophylactic treatment for Glanzmann Thrombasthenia with the potential to treat other debilitating rare bleeding disorders.