Highly Efficient Treatment for Killing Drug-Resistant Bacteria: Bacteriophage Therapy
Phages are composed of only nucleic acids encapsulated in a protein shell. They possess a very simple genetic structure and feature an alien-ship-like appearance. The process by which a prototypical bacteriophage invades bacteria can be divided into three stages: infection, reproduction, and maturation. Because of their reproductive characteristics, phages are classified into either virulent or temperate. Virulent phages prey on and lyse bacteria, while temperate phages initiate a symbiotic relationship with their host through lysogeny. The delicate and intriguing relationship between temperate phages and bacteria proves extremely promising for future research and invention. By harnessing biotechnology to accurately and controllably map this process, we can develop new treatments for bacterial infections to solve the current antibiotic resistance crisis. Challenging the pathogenicity of bacteria through Bacteriophage therapy could lead the way to improved antibiotic strategies.
Bacteriophages, serving as a therapeutic drug, pose many questions that warrant deep contemplation before transitioning to frontline clinical use. These include factors such as quality control standards for bacteriophage formulations, how to evaluate the safety of bacteriophages entering the body, administration methods, dosage forms, drug residues and side effects. Until these questions are fully answered or resolved, it's only to say that the lytic nature of bacteriophages provides beneficial clues for the treatment of clinical infectious diseases. In an era of rapid emergence and widespread dissemination of antibiotic-resistant bacteria, there is an urgent need to deepen our understanding of bacteriophage biology, establish scientific and effective bacteriophage pharmaceutical standards, so that bacteriophages could stand shoulder to shoulder with antibiotics, and reclaim their throne in combating bacterial diseases.
Bacteriophage Therapy Competitive Landscape
According to Patsnap Synapse, as of 8 Oct 2023, there are a total of 76 Bacteriophage therapy drugs worldwide, from 36 organizations, covering 10 targets, 48 indications, and conducting 41 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, targets, organizations, clinical trials, and clinical results related to this drug type.
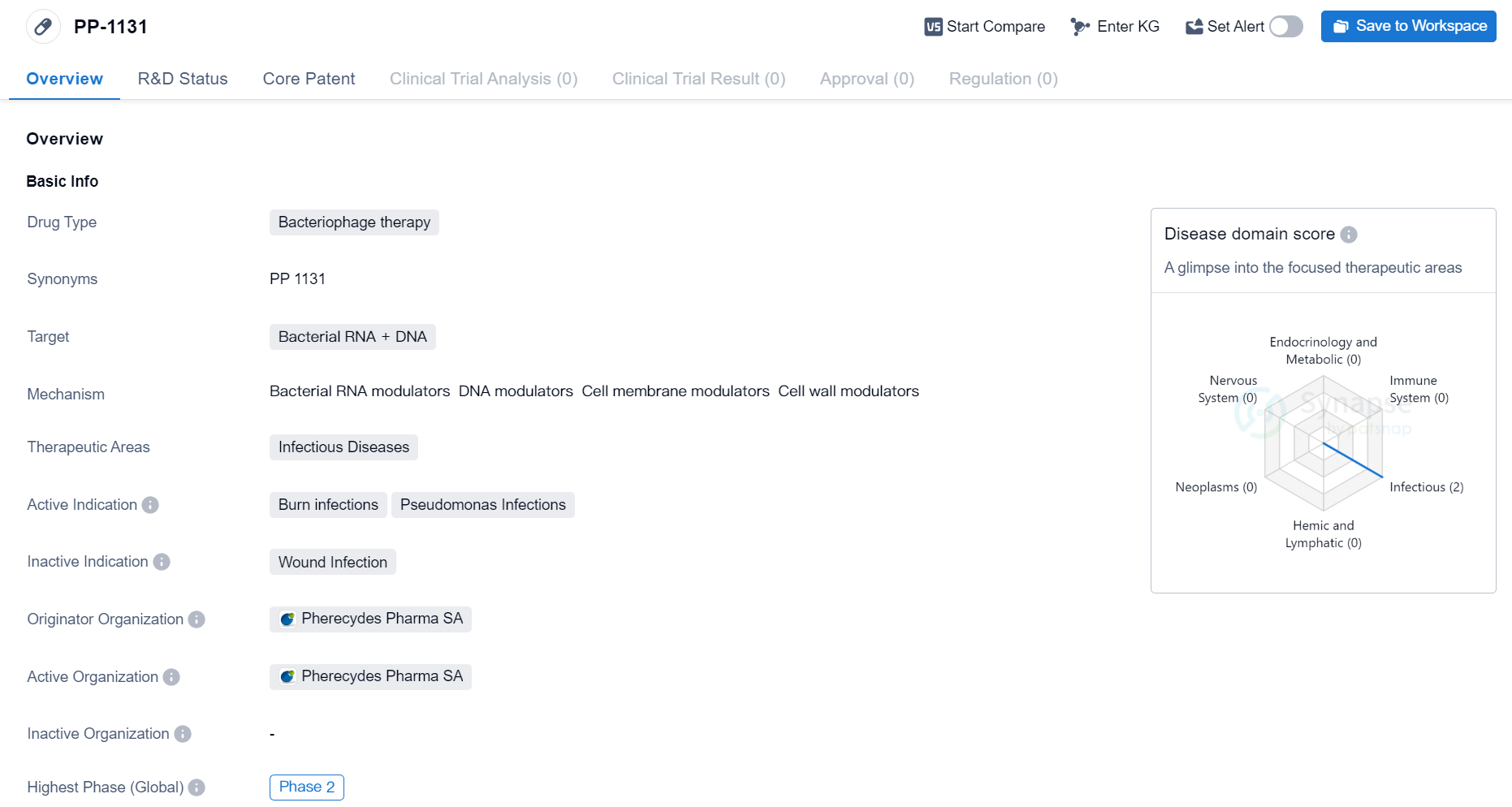
Phase II Clinical Trial of Bacteriophage Therapy Medicinal: PP-1131
PP-1131 is a bacteriophage therapy drug that targets bacterial RNA and DNA. It falls under the therapeutic area of infectious diseases and is specifically indicated for burn infections and Pseudomonas infections. The drug is being developed by Pherecydes Pharma SA, an originator organization in the field of biomedicine.
Bacteriophage therapy is a promising approach in the treatment of bacterial infections. Bacteriophages are viruses that specifically target and infect bacteria, leading to their destruction. By targeting bacterial RNA and DNA, PP-1131 aims to disrupt the genetic material of bacteria, ultimately killing them and treating the associated infections.
The therapeutic areas of infectious diseases encompass a wide range of conditions caused by pathogenic microorganisms. Burn infections and Pseudomonas infections are two specific indications for which PP-1131 is being developed. Burn infections are a common complication of severe burns, often caused by bacteria entering the damaged skin. Pseudomonas infections, on the other hand, are caused by the bacteria Pseudomonas aeruginosa and can affect various parts of the body, including the skin, lungs, and urinary tract.
Pherecydes Pharma SA is the originator organization behind PP-1131. As an originator, they are responsible for the development and initial research of the drug. The fact that PP-1131 has reached Phase 2 of clinical trials indicates that it has already undergone preliminary testing for safety and efficacy. Phase 2 trials involve a larger number of participants and aim to further evaluate the drug's effectiveness and side effects.
In summary, PP-1131 is a bacteriophage therapy drug developed by Pherecydes Pharma SA. It targets bacterial RNA and DNA and is intended for the treatment of burn infections and Pseudomonas infections. With its advancement to Phase 2 trials, PP-1131 shows promise in addressing these infectious diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
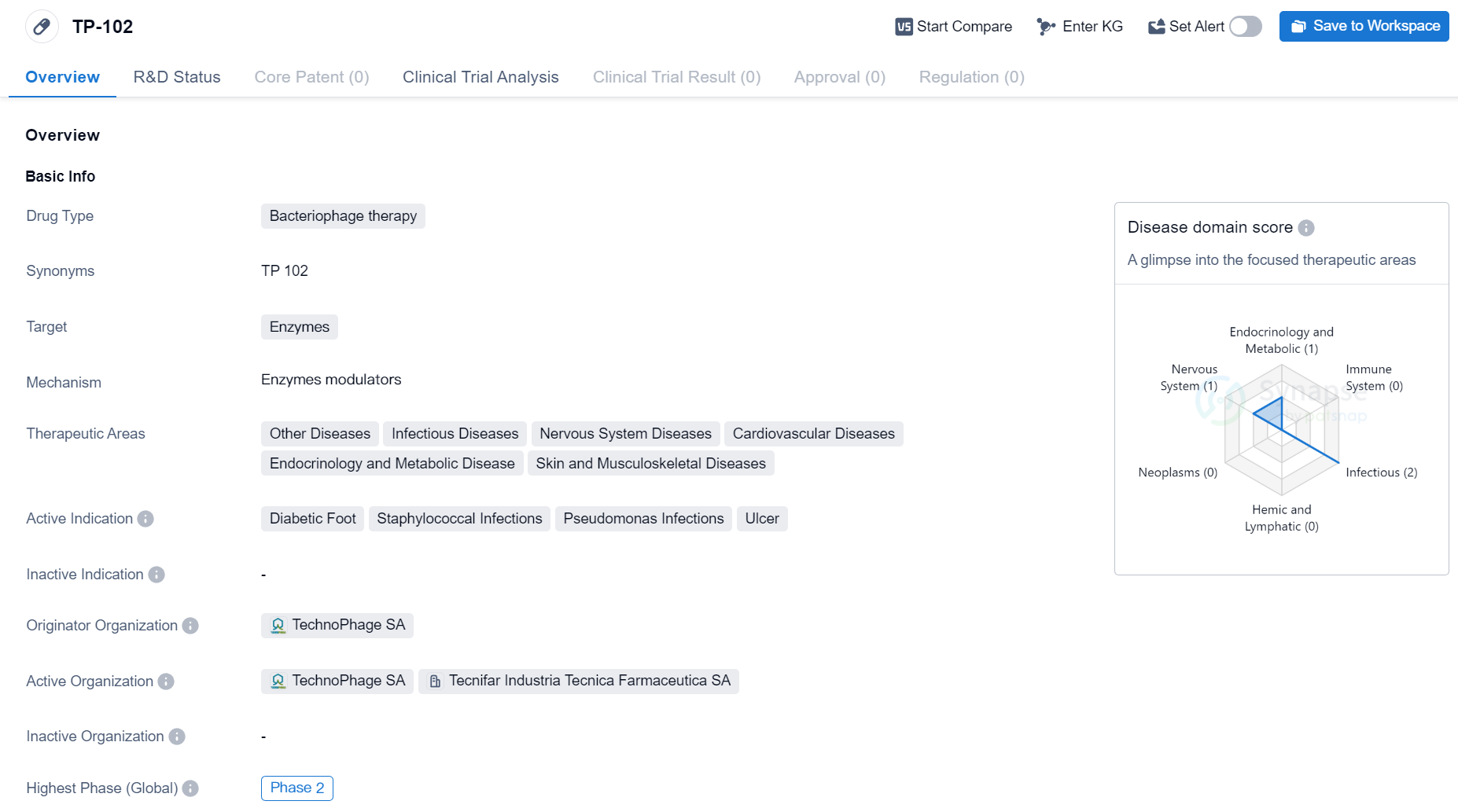
Phase II Clinical Trial of Bacteriophage Therapy Medicinal: TP-102
TP-102 is a bacteriophage therapy drug developed by TechnoPhage SA. Bacteriophage therapy involves the use of viruses called bacteriophages to target and kill specific bacteria. In the case of TP-102, the drug targets enzymes within bacteria.
The therapeutic areas that TP-102 aims to address are diverse and include other diseases, infectious diseases, nervous system diseases, cardiovascular diseases, endocrinology and metabolic diseases, as well as skin and musculoskeletal diseases. This suggests that TP-102 has the potential to be used in a wide range of medical conditions.
The drug's active indications are diabetic foot, staphylococcal infections, pseudomonas infections, and ulcers. Diabetic foot is a common complication of diabetes that can lead to serious infections and ulcers. Staphylococcal and pseudomonas infections are caused by specific bacteria and can be difficult to treat. Ulcers, which can occur in various parts of the body, are open sores that can be caused by a variety of factors.
TP-102 has reached Phase 2 of clinical development, which indicates that it has already undergone initial testing for safety and efficacy in humans. This is an important milestone in the drug development process, as it suggests that TP-102 has shown promising results in earlier stages of testing.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
As an objective summary, TP-102 is a bacteriophage therapy drug developed by TechnoPhage SA. It targets enzymes within bacteria and has the potential to be used in various therapeutic areas, including infectious diseases, cardiovascular diseases, and skin and musculoskeletal diseases. The drug's active indications are diabetic foot, staphylococcal infections, pseudomonas infections, and ulcers. TP-102 has reached Phase 2 of clinical development, indicating positive results in earlier stages of testing.