Hot Track in Disease Treatment: Mesenchymal Stem Cell Therapy
Mesenchymal stem cells (MSCs) have broad therapeutic potential for treating inflammation and degenerative diseases. Mesenchymal stem cells have been isolated and identified from many other sources of human tissues, widely distributed in virtually all tissues (including fetal and adult), such as bone marrow, blood, umbilical cord, placenta, fat, amnion, amniotic fluid, dental pulp, skin, menstrual blood, and so on.
Mesenchymal stem cell therapy works in both direct and indirect ways. The direct actions include homing, directed differentiation, multipotent differentiation, and paracrine effects. The indirect actions involve the bidirectional regulation of immune responses and inflammation, antioxidative stress, antifibrosis, antiapoptosis, hematopoiesis support, participation in or promotion of angiogenesis, activation of the proliferation and differentiation of endogenous stem cells, and the dedifferentiation of diseased tissue organs' normal cells, as well as anti-aging, and anti-scar formation.
The application of mesenchymal stem cell therapy in clinical practice is very broad, including the treatment of hematological diseases, neurological diseases, digestive diseases, cardiovascular diseases, respiratory diseases, skeletal system diseases, endocrine system diseases, immune system diseases, urogenital diseases, as well as anti-aging and immune enhancement. With the continuous investment in future scientific research, it is believed that there will be increasingly more difficult and complex diseases starting to explore the use of mesenchymal stem cells for treatment, and an increasing number of new drugs based on mesenchymal stem cells will be approved for clinical trials and even applications.
Mesenchymal Stem Cell Therapy Competitive Landscape
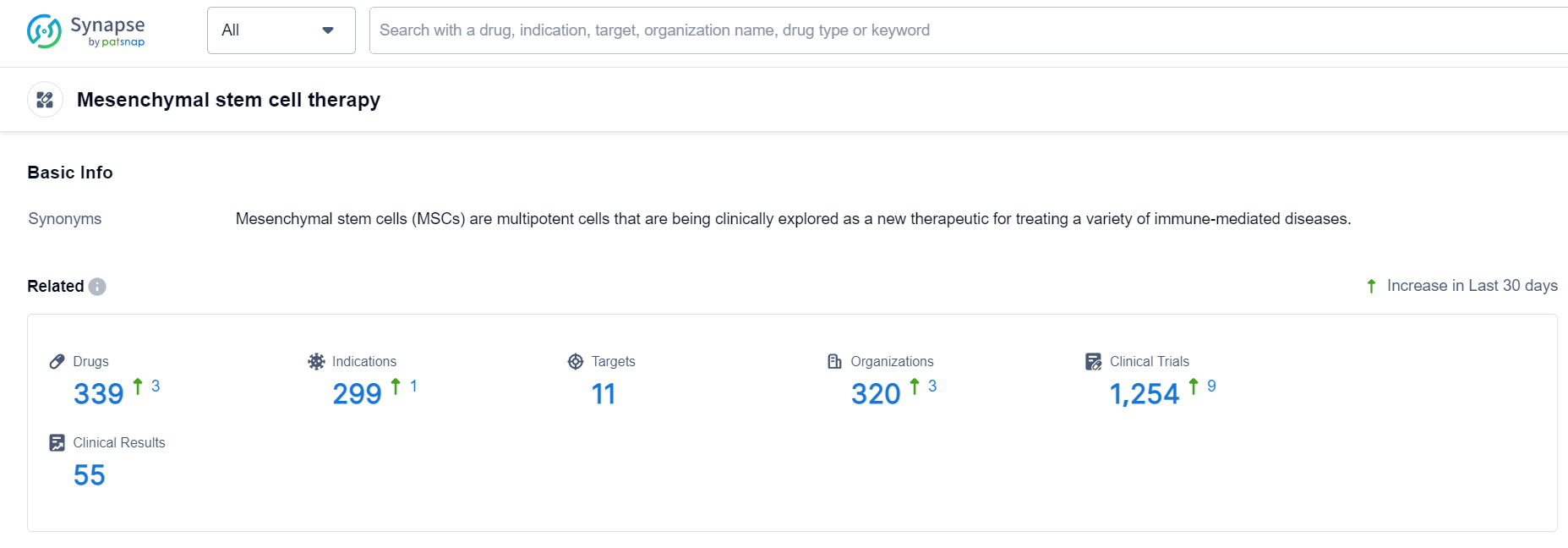
According to Patsnap Synapse, as of 8 Oct 2023, there are a total of 339 Mesenchymal stem cell therapy drugs worldwide, from 320 organizations, covering 11 targets, 299 indications, and conducting 1254 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, targets, organizations, clinical trials, and clinical results related to this drug type.
Phase II Clinical Trial of Mesenchymal Stem Cell Therapy Medicinal: ADR-001
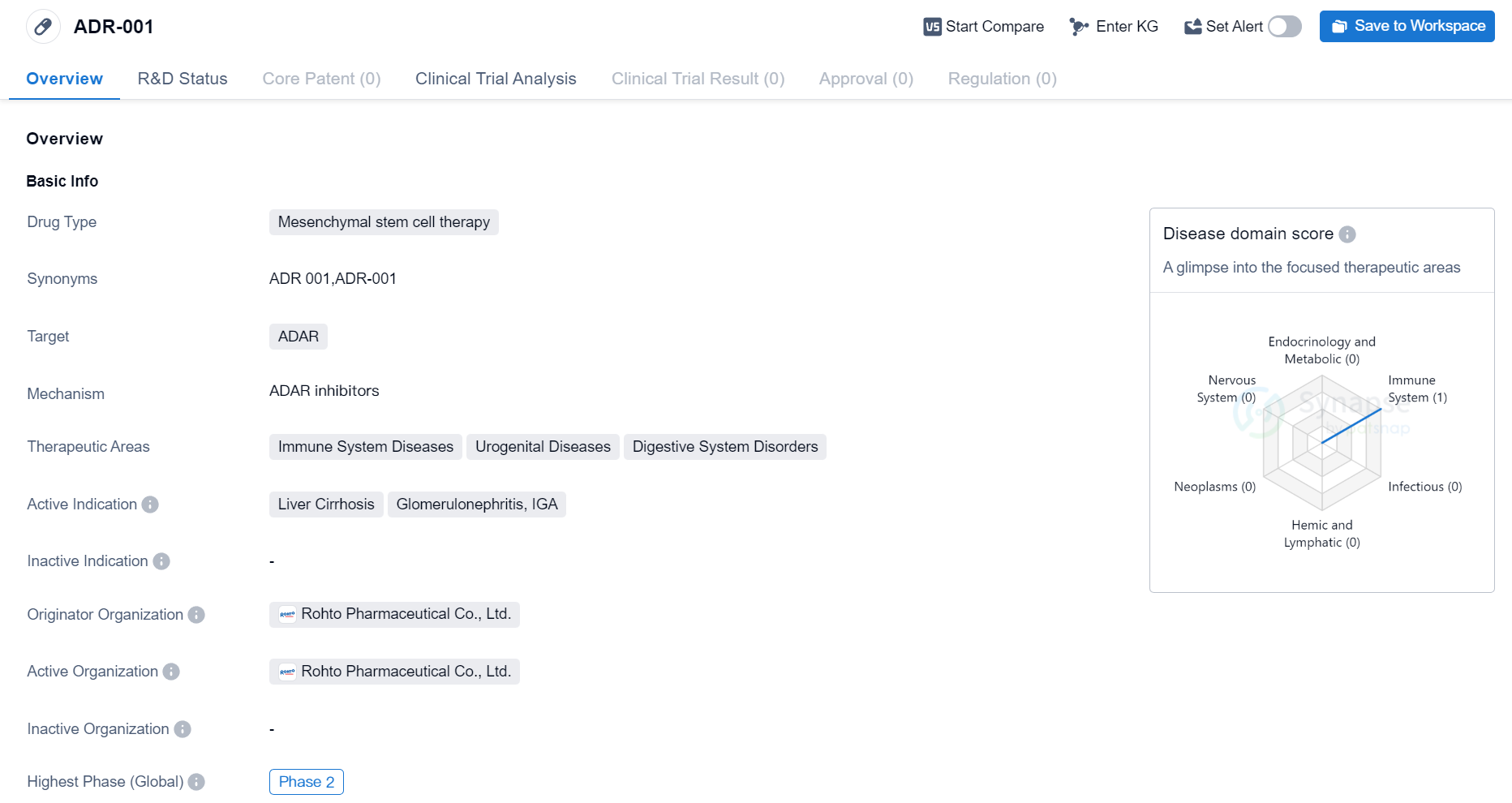
ADR-001 is a mesenchymal stem cell therapy drug that targets ADAR. Mesenchymal stem cells are multipotent cells that can differentiate into various cell types, including those found in the immune system, urogenital system, and digestive system. ADAR, or adenosine deaminase acting on RNA, is an enzyme involved in RNA editing.
The therapeutic areas for ADR-001 include immune system diseases, urogenital diseases, and digestive system disorders. Immune system diseases encompass a wide range of conditions that affect the body's immune response, such as autoimmune diseases and immunodeficiency disorders. Urogenital diseases refer to disorders affecting the urinary and reproductive systems, including conditions like urinary tract infections and infertility. Digestive system disorders involve issues with the gastrointestinal tract, such as inflammatory bowel disease and liver diseases.
The active indications for ADR-001 are liver cirrhosis, glomerulonephritis, and IGA. Liver cirrhosis is a chronic liver disease characterized by the replacement of healthy liver tissue with scar tissue, leading to impaired liver function. Glomerulonephritis is a group of kidney diseases that cause inflammation in the glomeruli, the tiny filters in the kidneys. IGA refers to immunoglobulin A, an antibody that plays a role in the immune response. Elevated levels of IGA can be associated with certain diseases, including kidney and liver disorders.
Rohto Pharmaceutical Co., Ltd. is the originator organization of ADR-001. They are responsible for the development and research of this drug. The highest phase of development for ADR-001 is Phase 2, indicating that it has undergone initial testing for safety and efficacy in a limited number of patients.
In summary, ADR-001 is a mesenchymal stem cell therapy drug developed by Rohto Pharmaceutical Co., Ltd. It targets ADAR and is being investigated for its potential in treating immune system diseases, urogenital diseases, and digestive system disorders. The active indications for ADR-001 include liver cirrhosis, glomerulonephritis, and IGA. Currently, ADR-001 is in Phase 2 of development, suggesting that further clinical trials are required to assess its effectiveness and safety.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Approved Mesenchymal Stem Cell Therapy Medicinal: Stempeutics
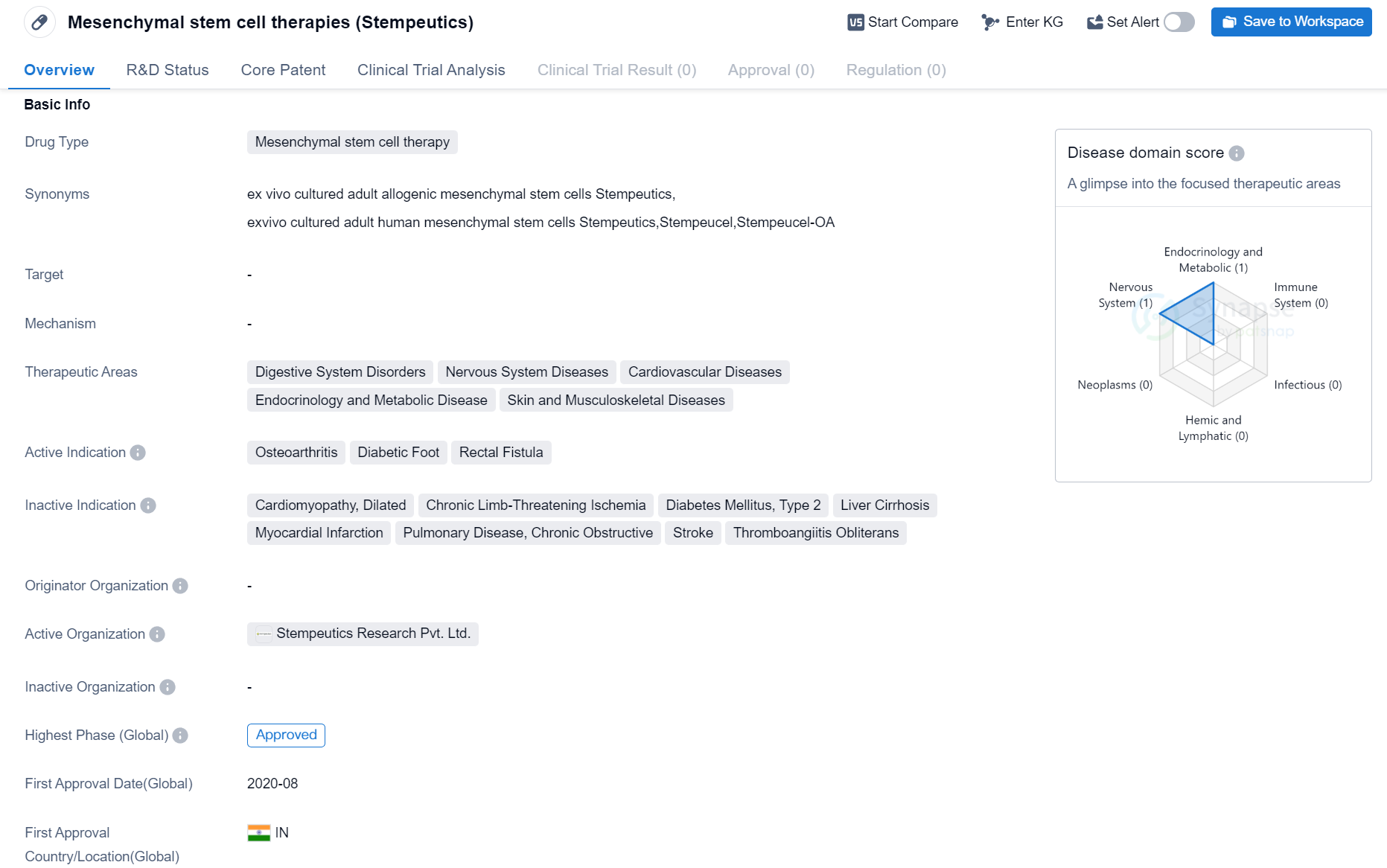
Mesenchymal stem cell therapies, specifically Stempeutics, are a type of drug used in the field of biomedicine. These therapies are designed to treat various medical conditions related to the digestive system, nervous system, cardiovascular system, endocrinology and metabolism, as well as skin and musculoskeletal diseases.
The active indications for Stempeutics include osteoarthritis, diabetic foot, and rectal fistula. These conditions are targeted by the drug to provide therapeutic benefits and alleviate symptoms associated with them.
In terms of its development stage, Stempeutics has reached the highest phase of approval, indicating that it has successfully undergone rigorous testing and evaluation. The first approval of Stempeutics was granted in August 2020 in India. This means that India was the first country/location to approve the drug for use in patients. This approval signifies that the regulatory authorities in India have reviewed the clinical data and determined that the drug meets the necessary standards for safety and effectiveness.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Overall, Stempeutics is a mesenchymal stem cell therapy that has been approved for the treatment of osteoarthritis, diabetic foot, and rectal fistula. It has shown promising results in clinical trials and has received approval in India. The drug holds potential for addressing various medical conditions related to the digestive system, nervous system, cardiovascular system, endocrinology and metabolism, as well as skin and musculoskeletal diseases.