How to find the chemical modification of Lumasiran?
Lumasiran, developed by Alnylam Pharmaceuticals, is a revolutionary RNAi therapeutic that targets hydroxyacid oxidase 1 (HAO1) mRNA, which is involved in the synthesis of glycolate oxidase (GO), an enzyme that catalyzes the production of glycolate, a precursor to oxalate. This innovative drug was approved by the FDA in November 2020 for the treatment of primary hyperoxaluria type 1 (PH1), a rare genetic disorder characterized by excessive production of oxalate that can lead to kidney stones and kidney damage.
Summary of Research Progress of Lumasiran
Lumasiran works by targeting hydroxyacid oxidase 1 (HAO1) mRNA, thereby inhibiting the synthesis of glycolate oxidase (GO), an enzyme involved in the production of glycolate, a precursor to oxalate.
Notably, lumasiran has shown promising results in clinical trials, particularly the Phase 3 ILLUMINATE-A study (NCT03681184), which demonstrated significant reductions in urinary oxalate excretion, bringing levels closer to or within the normal range for many patients treated with the drug. These findings underscore the potential of lumasiran to address the underlying pathology of PH1 effectively.
The drug's mechanism of action involves RNA interference (RNAi), a biological process that uses small interfering RNAs (siRNAs) to silence specific genes, in this case, HAO1. This innovative approach allows for precise targeting of the gene responsible for excessive oxalate production without affecting other metabolic processes.
The approval of lumasiran by the U.S. Food and Drug Administration (FDA) in November 2020 marked a significant milestone in the treatment of PH1, offering a novel therapeutic option for patients who previously had limited treatment choices. Lumasiran is administered subcutaneously and is designed to be self-administered, providing patients with the convenience and potential to improve treatment adherence.
Following its approval, lumasiran has been introduced to the global market, with marketing efforts focusing on reaching those diagnosed with PH1 and educating healthcare providers about the benefits of this new treatment option. The drug has received orphan drug status and breakthrough therapy designation, highlighting its importance in treating a rare and serious condition.
Alnylam Pharmaceuticals continues to support the global rollout of lumasiran through partnerships and collaborations aimed at expanding access to the drug worldwide. Additionally, the company is exploring the potential of lumasiran in combination with other therapies to further enhance its effectiveness in managing PH1 and related complications.
Sequence Information and Characteristics of Lumasiran
Lumasiran works by specifically binding to HAO1 mRNA and promoting its degradation, thereby reducing the synthesis of GO enzyme. With less GO enzyme available, the production of glycolate, and subsequently oxalate, is diminished. This mechanism makes lumasiran a disease-modifying therapy rather than a mere symptomatic treatment.
Lumasiran is a small interfering RNA (siRNA) that is chemically modified to enhance its stability and effectiveness. It is conjugated to N-acetylgalactosamine (GalNAc), which facilitates its uptake by hepatocytes, the primary site of oxalate production. This specific targeting is crucial for the drug's efficacy and contributes to its favorable safety profile.
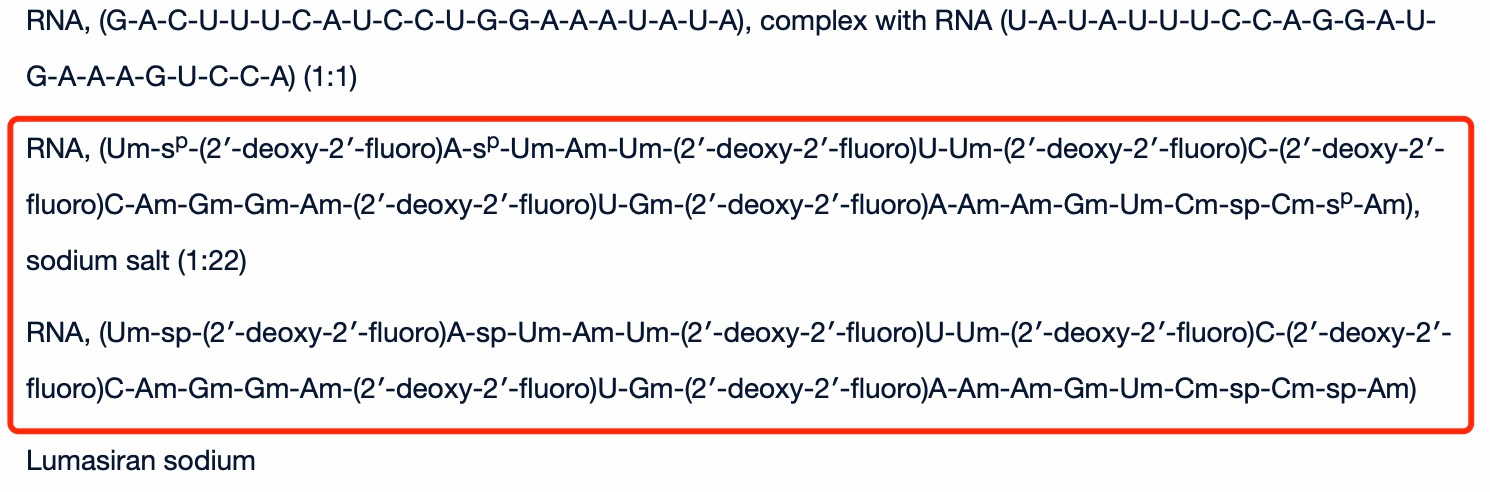
Key characteristics of lumasiran include its chemical modifications and GalNAc conjugation. Lumasiran utilizes Alnylam's Enhanced Stabilization Chemistry (ESC)-GalNAc technology, which enhances the stability and potency of the siRNA molecule. The conjugation of N-acetylgalactosamine (GalNAc) facilitates the uptake of the drug by hepatocytes, the primary cells responsible for oxalate production. This targeted delivery to the liver ensures high efficacy while minimizing systemic exposure, contributing to its favorable safety profile.
In terms of molecular structure, lumasiran is a double-stranded RNA molecule with specific nucleotide sequences that are complementary to the HAO1 mRNA. Its molecular formula is C530H669F10N173O320P43S6Na43, with a molecular weight of 17,286 Daltons. Lumasiran is administered subcutaneously and is formulated as a clear, colorless to slightly yellow solution intended for subcutaneous injection.
The administration of lumasiran is designed to be convenient and manageable, with a regimen that includes initial loading doses followed by maintenance dosing, tailored to individual patient needs. These features, combined with the specificity of its mechanism of action and the convenience of self-administration, make lumasiran a significant advancement in the treatment of PH1, offering hope to patients who previously had limited therapeutic options.
Chemical Modification and Action of Lumasiran
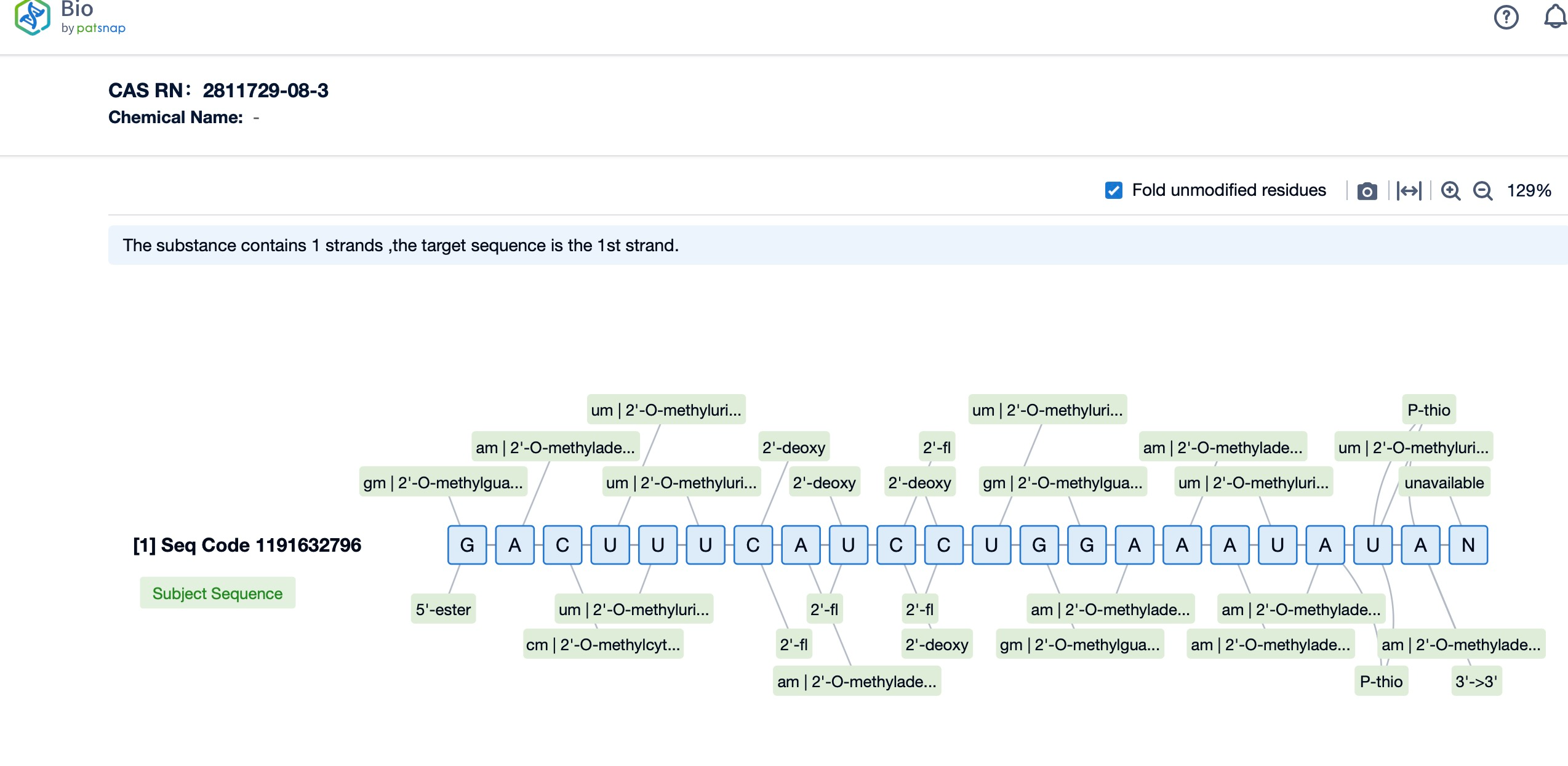
The chemical modifications of lumasiran include the conjugation to GalNAc, which is a key component of its delivery system. This conjugation allows for targeted delivery to the liver, where it can effectively reduce the production of the GO enzyme. The modifications also increase the drug's resistance to degradation, extending its duration of action.
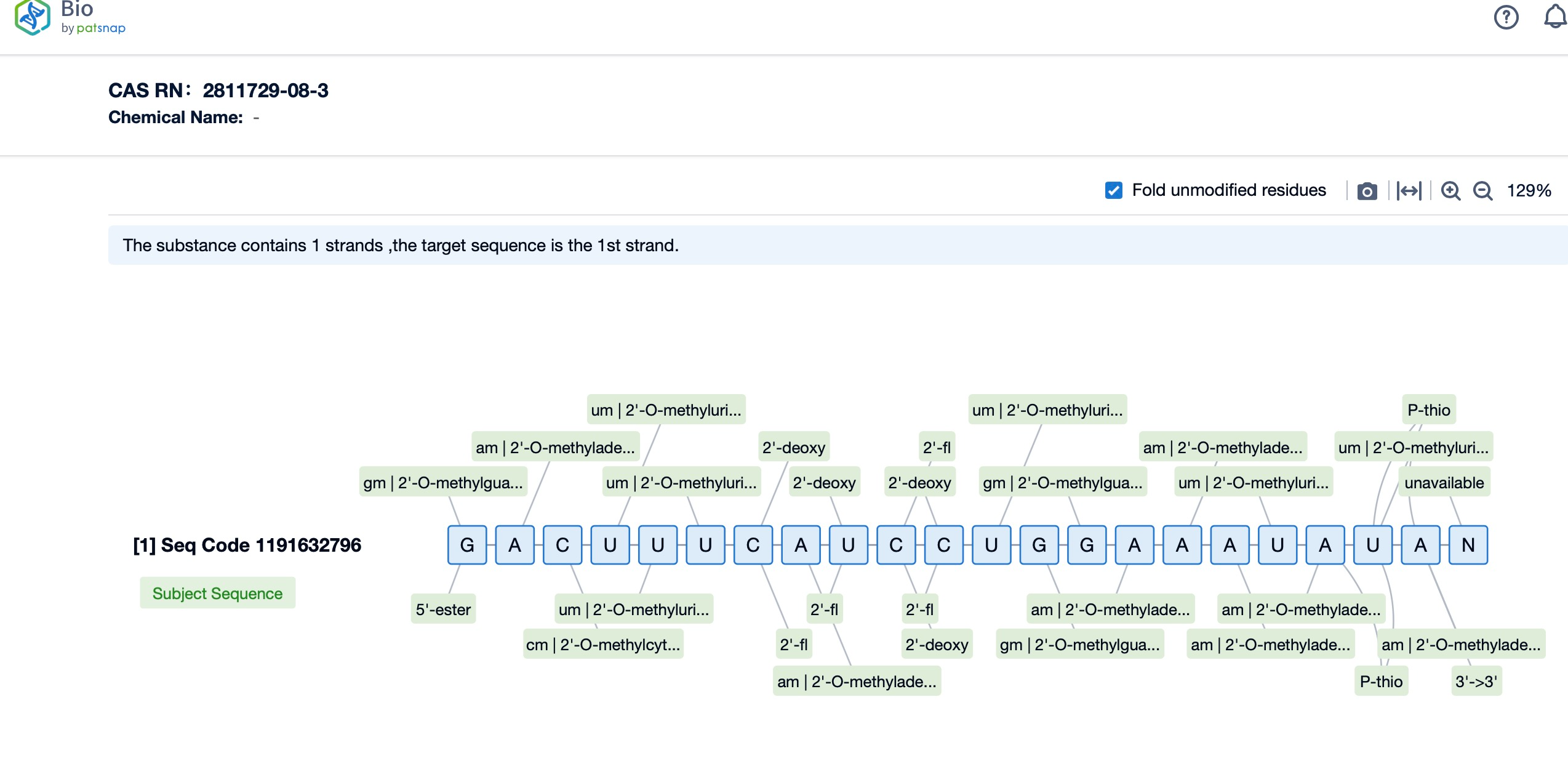
Specifically engineered with Enhanced Stabilization Chemistry (ESC) modifications, lumasiran exhibits increased resistance to nucleases and improved pharmacokinetic properties, allowing for a durable response with less frequent dosing. The drug's siRNA duplex is conjugated to N-acetylgalactosamine (GalNAc), a ligand that binds to the asialoglycoprotein receptor (ASGPR) on the surface of hepatocytes, facilitating the efficient uptake of lumasiran by liver cells. Once internalized, lumasiran integrates into the RNA-induced silencing complex (RISC), where it specifically targets and degrades hydroxyacid oxidase 1 (HAO1) mRNA, thereby inhibiting the synthesis of glycolate oxidase (GO), a key enzyme in oxalate production. This targeted RNA interference (RNAi) mechanism not only curbs the overproduction of oxalate but also maintains a favorable safety profile by limiting off-target effects and ensuring the specificity of action. Consequently, lumasiran represents a major leap forward in the management of PH1, offering a precisely targeted therapy that addresses the root cause of the disease while minimizing potential adverse reactions.
Summary and Prospect
In summary, Lumasiran represents a breakthrough in the treatment of PH1, offering a targeted approach to reducing oxalate production and potentially altering the disease's progression. Its approval and market introduction have been supported by robust clinical trial data and a deep understanding of the disease's pathology. Looking forward, lumasiran is poised to become a cornerstone treatment for PH1, with ongoing research and development efforts aiming to expand its applications and further improve the lives of patients living with this rare disease.
How to find the chemical modification of all siRNAs?
In Patsnap Bio, you can find the sequence and latest research and development advances of all siRNAs.
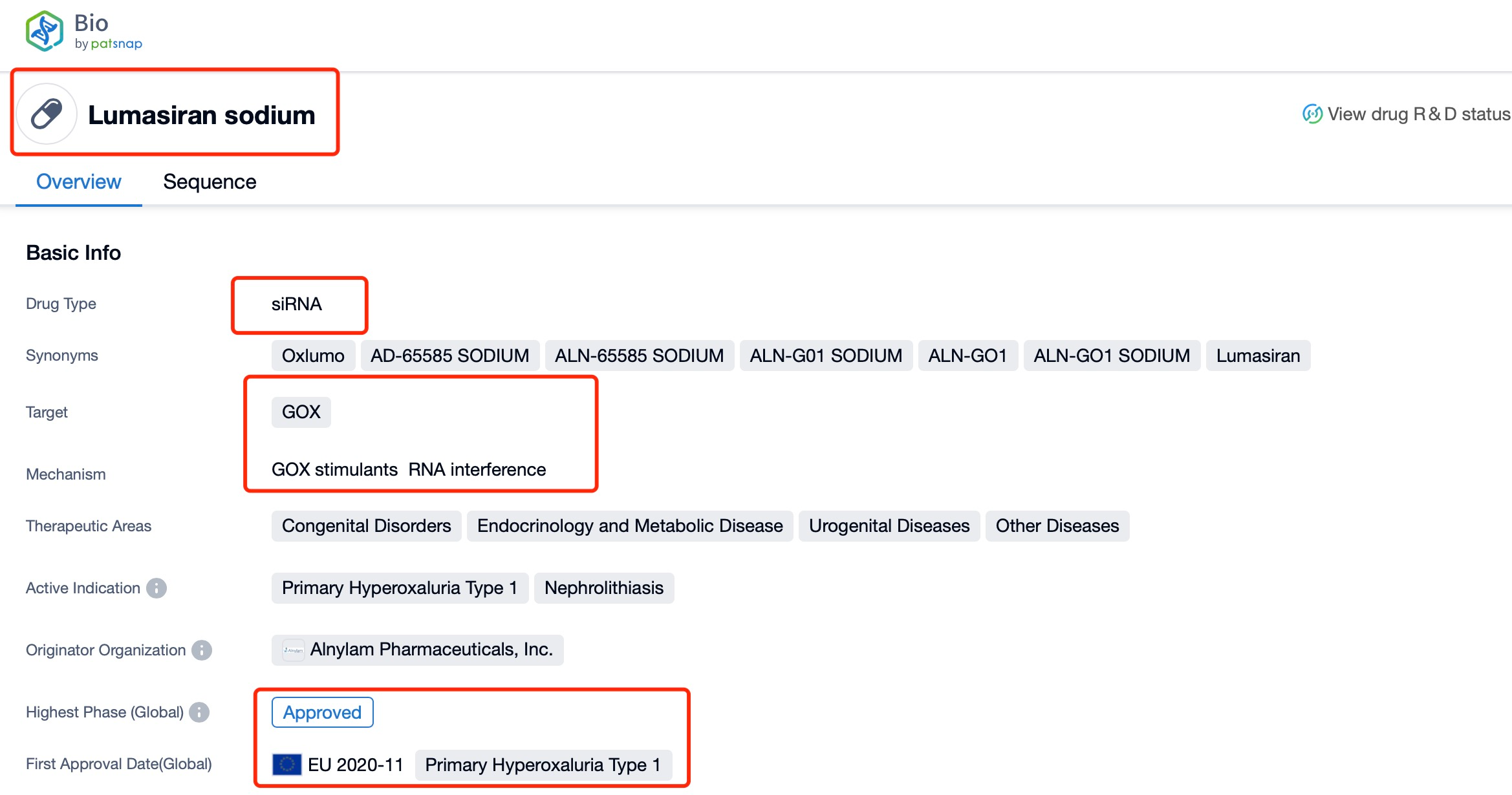
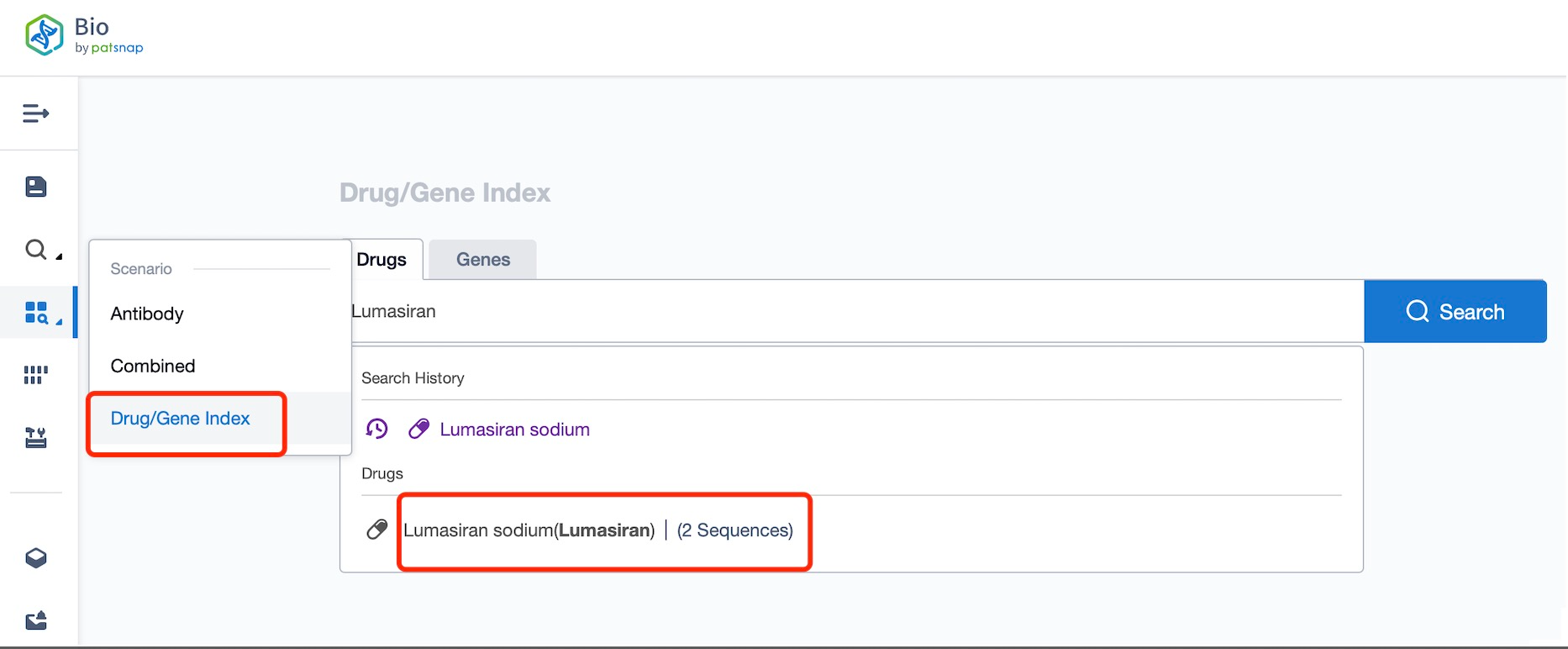
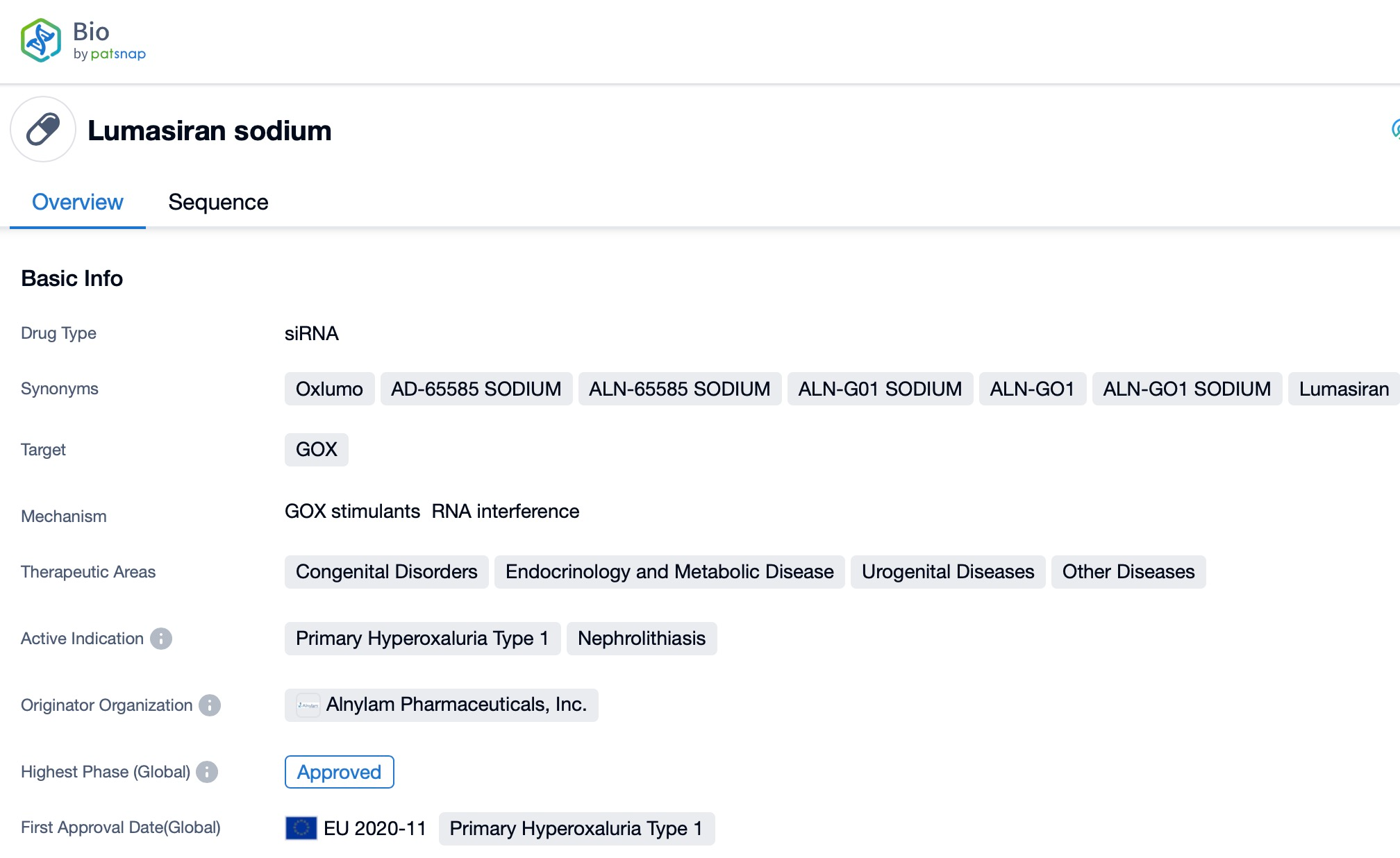
Taking Lumasiran as an example, first click on the Drug/Gene Index on the Patsnap Bio homepage. Here you can search for sequence information by drug and gene names. Enter 'Lumasiran' in the search box and click to view the details. On the details page, you can find the basic information and research progress of lumasiran.
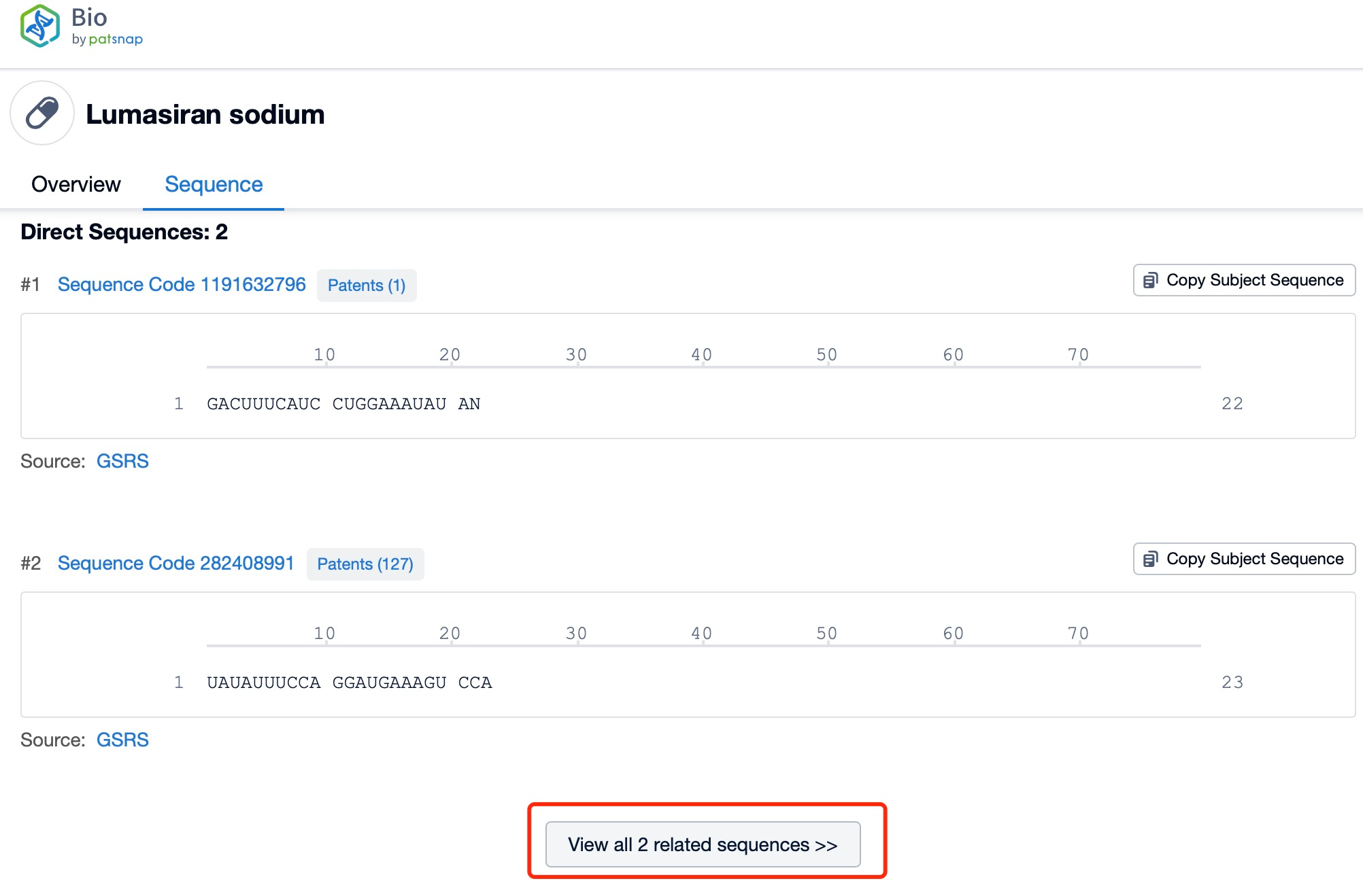
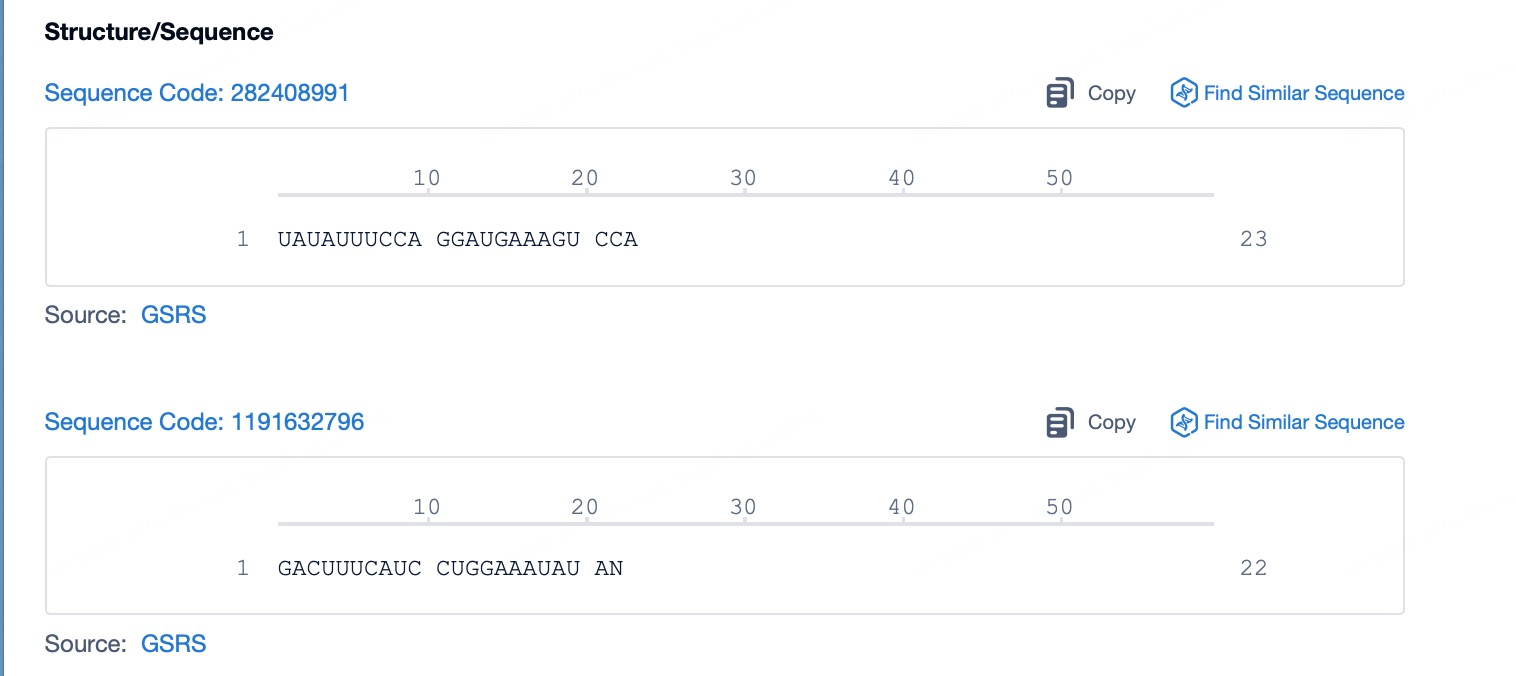
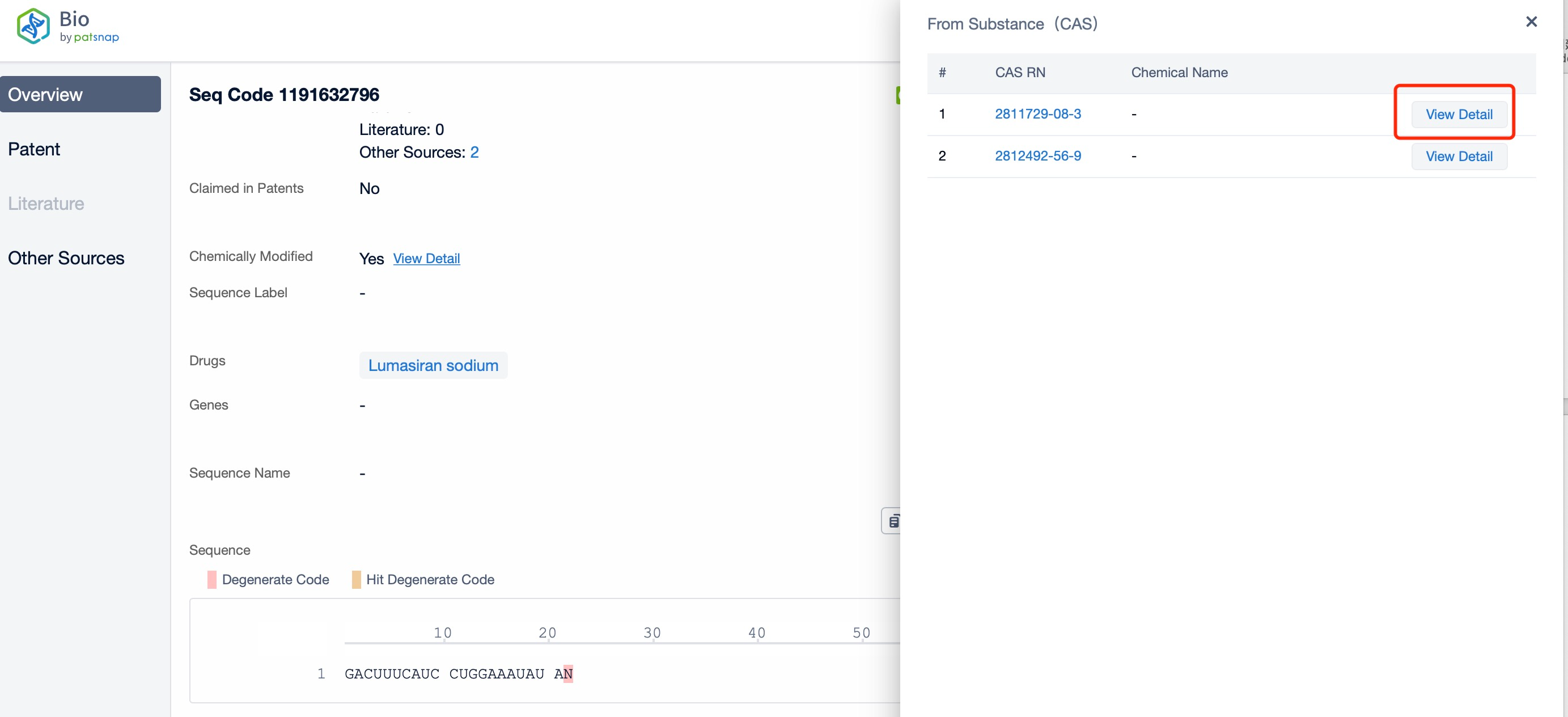
Click "View all related sequences" below the sequence information to search for and retrieve all biological sequences similar to this information.
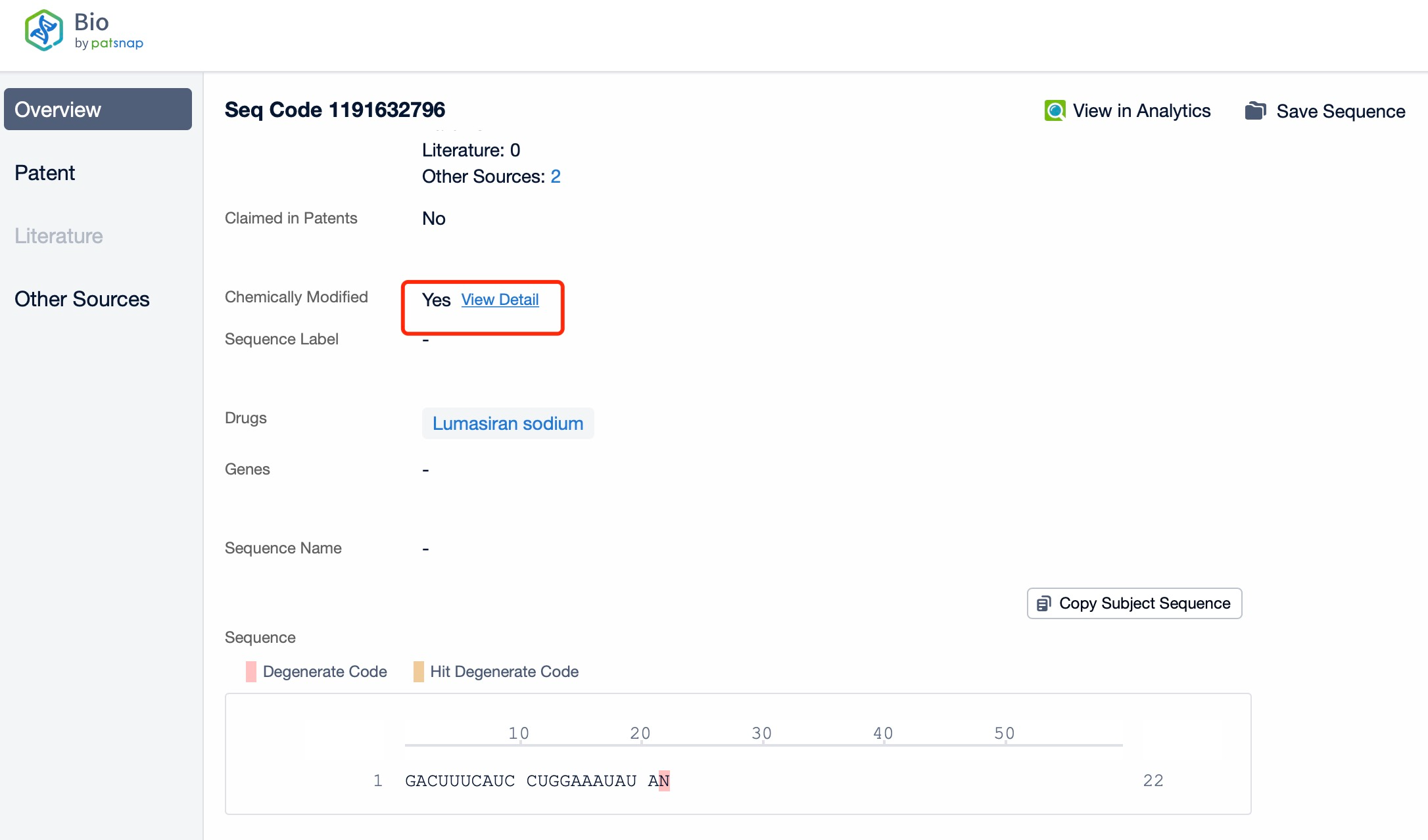
Clicking on the sequence name will provide you with all the basic information of that sequence. 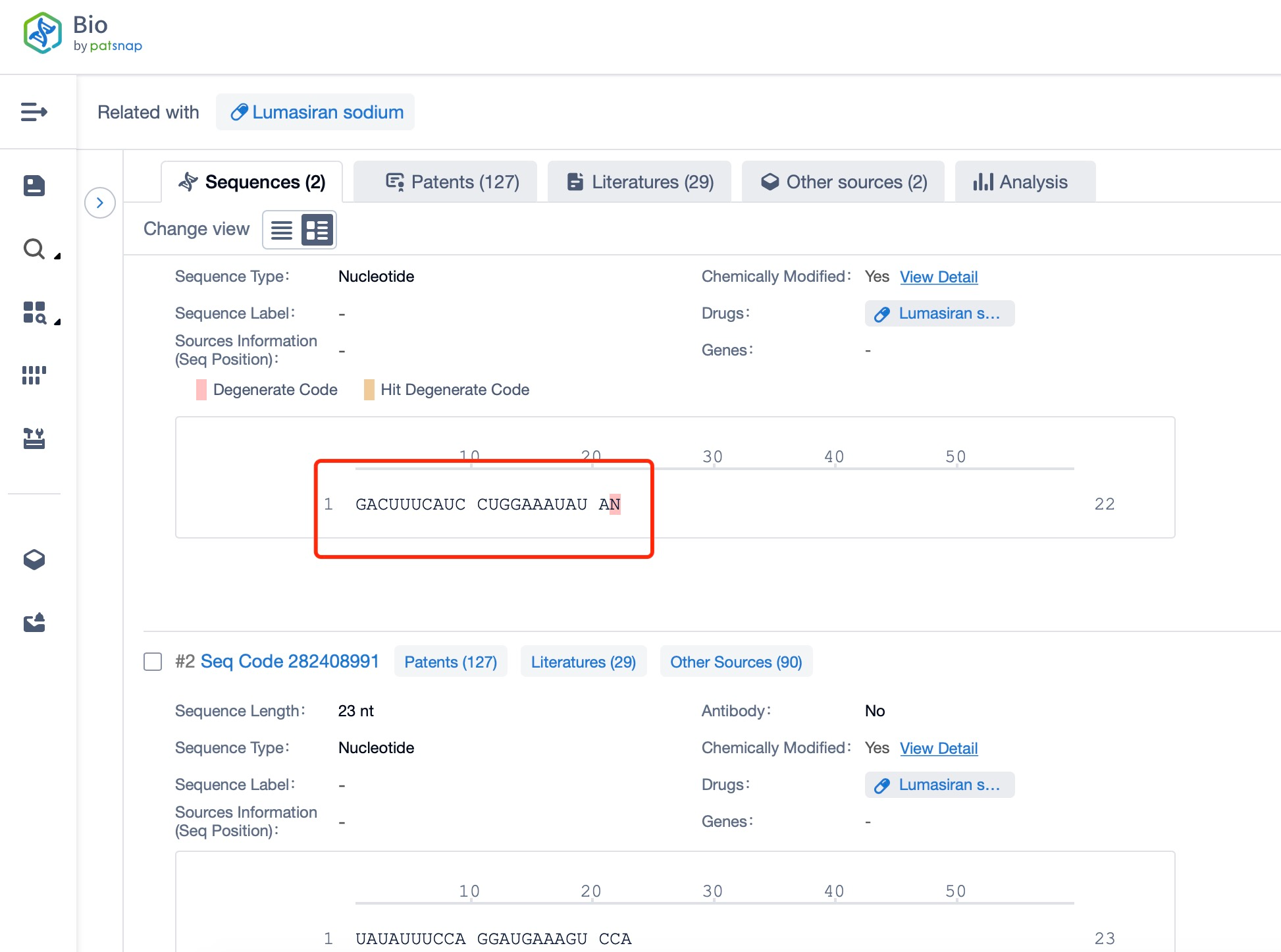
Additionally, a visual diagram of the sequence's chemical modifications is available for immediate access.
Patsnap Bio helps you turn weeks into minutes with cutting-edge AI-enabled tools built to master the complexities of sequence retrieval and automate IP analysis with precision and ease.